
You often use lithium-ion batteries in devices like smartphones, laptops, and electric vehicles. The 3.7v standard voltage comes from the chemistry inside each lithium-ion battery. Lithium ions move between the graphite anode and the lithium cobalt oxide cathode during charging and discharging. This process creates a voltage difference, giving lithium-ion batteries their 3.7v standard voltage. The unique design and chemistry of lithium-ion batteries make them popular for rechargeable applications in consumer electronics, offering high energy, safety, and long-lasting performance.
3.7V Standard Voltage in Lithium-Ion Batteries

Nominal Voltage Meaning
You may wonder what the term “nominal voltage” means when you look at lithium-ion batteries. Nominal voltage is the average voltage a battery cell maintains during its normal use. It is not the highest or lowest voltage the cell can reach. Instead, it gives you a simple way to understand how the battery will perform in most situations. For lithium-ion batteries, the nominal voltage is usually 3.7v. This value helps you compare different batteries and makes it easier for engineers to design devices that use lithium-ion battery cells.
"(《世界人权宣言》) 3.7v standard voltage comes from the chemistry inside the lithium-ion battery. The materials used in the battery, such as the lithium cobalt oxide cathode and graphite anode, set this average voltage. When you use a lithium-ion battery, the voltage changes as the battery charges and discharges. The nominal voltage acts as a reference point, so you know what to expect during regular use. This standard makes it easier for you to choose the right battery for your devices and ensures that your electronics work safely and efficiently.
小贴士 Nominal voltage is a helpful guide for both consumers and engineers. It simplifies the complex behavior of lithium-ion batteries into a single, easy-to-understand number.
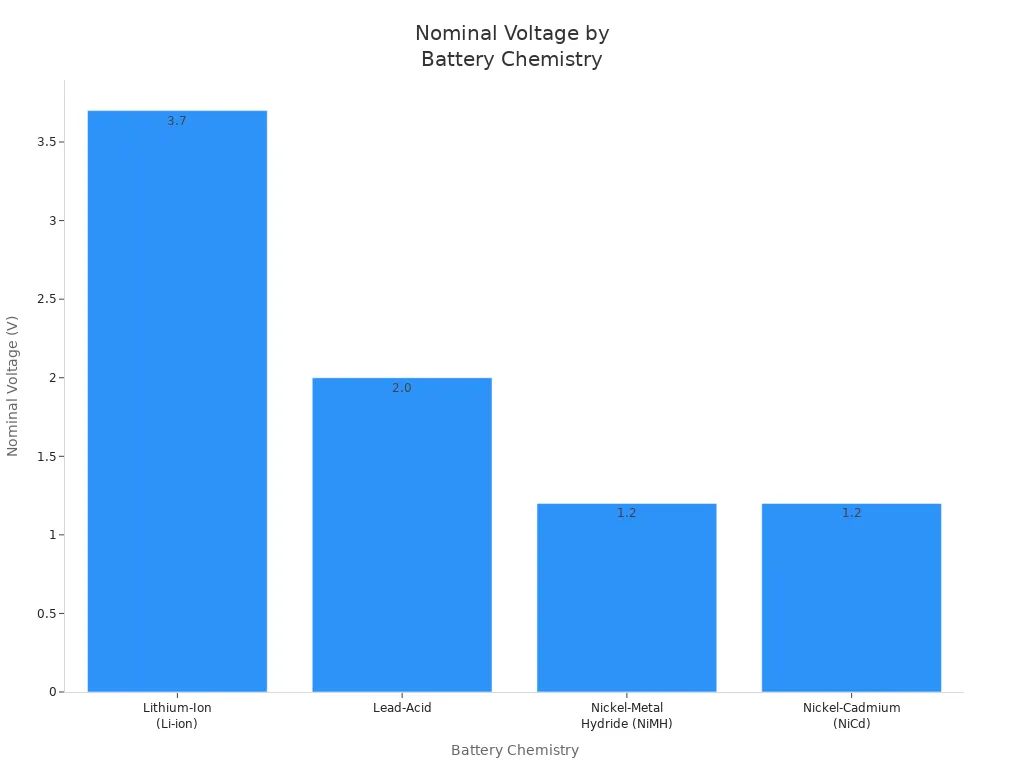
You can see how the 3.7v standard voltage compares to other rechargeable battery types in the table below:
| 电池化学 | Nominal Voltage per Cell | 典型应用 |
|---|---|---|
| 锂离子(Li-Ion) | 3.7 V | Smartphones, laptops, electric vehicles |
| 铅酸 | 2.0 V | Automotive batteries, backup power |
| 镍氢(NiMH) | 1.2 V | Rechargeable AA/AAA batteries, power tools |
| 镍镉 (NiCd) | 1.2 V | Power tools, emergency lighting |
You notice that lithium-ion batteries have a higher nominal voltage than other rechargeable batteries. This higher voltage allows lithium-ion batteries to deliver more energy and power in a smaller, lighter package. That is why you find lithium-ion batteries in so many modern applications, from smartphones to electric vehicles.
Voltage Range and Operation
When you charge a lithium-ion battery, the voltage rises to about 4.2 volts. As you use the battery, the voltage slowly drops. When the battery is almost empty, the voltage reaches about 3.0 volts. The 3.7v standard voltage is the average value between these two points. This average is important because it reflects the typical voltage you see during most of the battery’s discharge cycle.
下面的表格显示了 voltage range for a typical lithium-ion battery cell:
| Battery Parameter | Voltage (V) | 说明 |
|---|---|---|
| 额定电压 | 3.7 | Standard operating voltage of the cell |
| Full Charge Voltage | 4.2 | Maximum voltage when fully charged |
| Discharge Cut-off Voltage | 3.0 | Minimum safe voltage before discharge stops |
| Charging Voltage (with PCB) | 4.8 – 5.2 | Voltage range for charging with protection |
| Charging Voltage (no PCB) | ~4.2 | Maximum charging voltage without protection |
During use, the voltage of a lithium-ion battery does not stay at 3.7 volts all the time. Instead, it starts high when fully charged and drops as you use the battery. The nominal voltage gives you a good idea of the average voltage you will see during normal operation. This helps you predict how much energy you can get from the battery before you need to recharge it.
The 3.7v standard voltage is not just a random number. It comes from the chemistry of the lithium-ion battery. The materials inside the battery set the maximum and minimum voltages. The average of these values gives you the 3.7v standard voltage. This standard helps you use lithium-ion batteries safely and efficiently in many rechargeable applications.
You find lithium-ion batteries in many devices because of their high energy density and reliable voltage. These batteries power smartphones, laptops, electric vehicles, and even medical devices. The 3.7v standard voltage makes it easier for manufacturers to design products that are safe, efficient, and compatible with a wide range of rechargeable battery-powered devices.
Battery Chemistry and Design

Lithium Cobalt Oxide Cathode
You find that the chemistry of lithium-ion batteries starts with the choice of cathode material. The most common cathode in lithium-ion battery design is lithium cobalt oxide (LiCoO₂, or LCO). This material sets the voltage and performance of the battery. The layered crystal structure of LCO lets lithium ions move in and out during charging and discharging. This movement is called intercalation and deintercalation. The redox potential of cobalt in LCO gives the battery a high voltage, close to 4.55 volts compared to lithium metal.
- The layered structure of LCO supports stable lithium-ion movement, which is key for high energy density and high specific energy.
- LCO can reach high voltages, but pushing the voltage too high causes phase transitions. These changes in structure can lead to cracks and loss of performance.
- To keep the battery safe and long-lasting, you need to avoid these phase transitions. Doping the material with elements like magnesium or adding surface coatings helps prevent damage and allows for higher voltage operation.
- The chemistry and structure of LCO set the voltage window for lithium-ion batteries. Degradation at high voltage limits the practical voltage and capacity you can use.
Other cathode materials also play a big role in lithium-ion battery design. Each material gives a different voltage and performance. You can see the differences in the table below:
| Cathode Material | 标称电压 (V) | Typical Operating Voltage Range (V/cell) |
|---|---|---|
| 磷酸铁锂(LFP) | 3.2 – 3.3 | 2.5 – 3.65 |
| 氧化锰锂(LMO) | ~3.7 | 3.0 – 4.2 |
| 氧化钴锂(LCO) | 3.6 | 3.0 – 4.2 |
| 镍锰钴氧化锂(NMC) | 3.6 – 3.7 | 3.0 – 4.2 (or higher) |
| 镍钴铝氧化锂(NCA) | 3.6 | 3.0 – 4.2 |
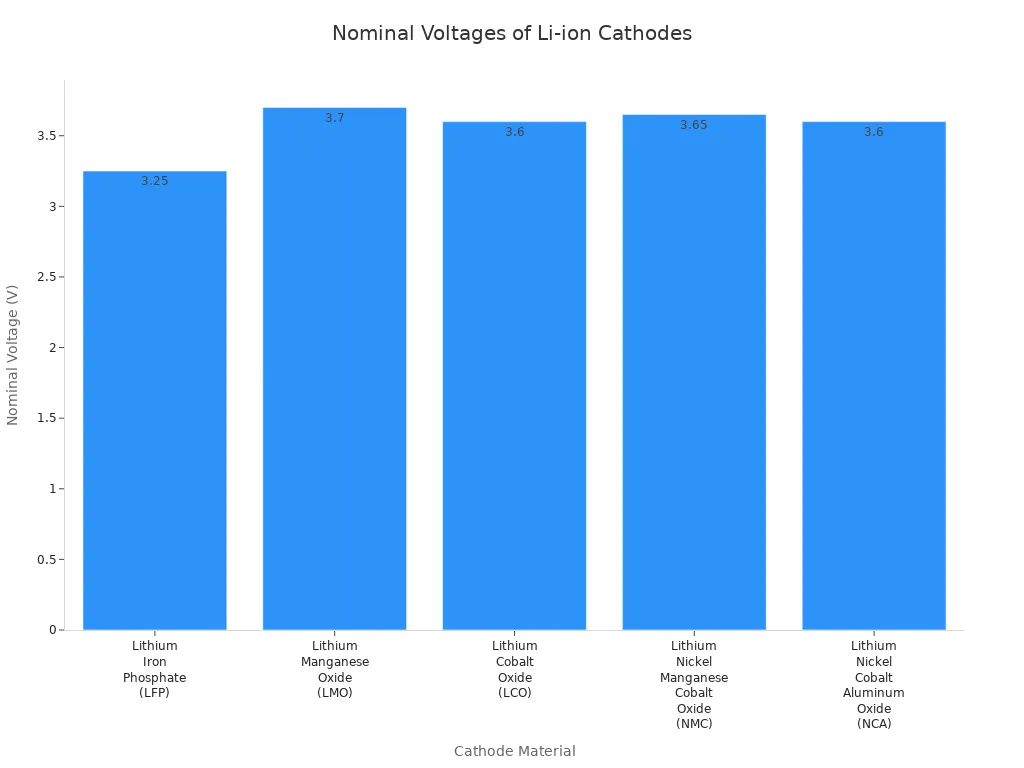
You notice that each cathode material changes the voltage and energy density of the battery. LFP gives you a lower voltage but better safety. LCO and NMC offer higher voltage and higher energy density, which is important for rechargeable applications that need high energy, like smartphones and electric vehicles. The chemistry of the cathode decides how much energy you can store and how safe the battery will be.
Role of Anode and Internal Resistance
The anode in lithium-ion batteries also affects the voltage and performance. Most lithium-ion battery designs use graphite as the anode. The size and shape of graphite particles change how fast lithium ions can move. Smaller particles let you charge and discharge faster, but they also create more heat and can lower the battery’s capacity over time. Higher density graphite reduces the space for lithium ions, which can help with stability but may slow down the movement of ions.
- Graphite’s voltage is close to lithium metal, which helps the battery reach a high voltage and high energy density.
- If the anode is not balanced with the cathode, you can get lithium plating. This happens when lithium metal forms on the anode, causing voltage spikes and safety risks.
- The chemistry of the anode, the electrolyte, and the ratio of anode to cathode all affect how the battery works and how safe it is.
Some new lithium-ion batteries use silicon anodes. Silicon can store more lithium, giving you higher energy density. Silicon anodes have higher electrical resistance, but their special structure helps ions move quickly. This reduces internal losses and keeps the voltage stable during use.
Internal resistance is another key part of battery design. Every lithium-ion battery has some resistance inside. When you use the battery, this resistance causes a voltage drop. The voltage you see at the battery’s terminals is lower than the open circuit voltage. The formula for this is:
Ucell = Uocv − ηdiff − ηch,tr − IRi
- Ucell is the voltage you get when the battery is working.
- Uocv is the voltage when the battery is not in use.
- ηdiff and ηch,tr are extra losses from chemical reactions.
- IRi is the voltage drop from internal resistance.
When you use a device that needs a lot of power, like a smartphone or a power tool, the internal resistance causes the voltage to drop even more. This can make your device shut down early or show a low battery warning. Internal resistance also turns some energy into heat, which lowers efficiency and can speed up battery aging.
请注意: Lower internal resistance means better performance, higher energy, and longer battery life. Good battery design keeps resistance low to improve safety and efficiency.
You see that the chemistry of both the cathode and anode, along with internal resistance, sets the voltage, energy density, and safety of lithium-ion batteries. Careful battery design lets you get the most energy and longest life from your rechargeable batteries in many applications.
Performance and Safety in Lithium Batteries
能量密度和容量
You rely on lithium-ion battery technology because it offers high energy density and strong performance. The 3.7V standard voltage comes from the chemistry between the lithium cobalt oxide cathode and graphite anode. This voltage lets lithium batteries store more energy in a smaller space. You see this advantage in many rechargeable electronics, electric vehicles, and even the automotive sector.
Lithium-ion batteries reach energy densities of 150–250 Wh/kg. This is much higher than older rechargeable batteries. The high energy density means you can use lighter batteries in your devices and vehicles. You get longer run times for your electronics and more miles for your electric vehicles. The 3.7V design also supports high specific energy, which is important for high performance in automotive and electronics applications.
- Lithium-ion batteries with 3.7V nominal voltage deliver more energy per unit weight.
- Lower voltage chemistries, like lithium iron phosphate, offer better safety but less energy density.
- The 3.7V standard balances energy density, safety, and stability for a wide range of applications.
小贴士 High energy density in lithium-ion battery design helps you enjoy compact, long-lasting rechargeable devices.
Charging, Cycle Life, and Safety
Charging lithium-ion batteries to higher voltages can give you more energy for each cycle. However, this comes with trade-offs. If you charge above the recommended voltage, you risk faster battery aging and reduced cycle life. High voltage charging can cause lithium plating on the anode. This leads to capacity loss and can create safety hazards, such as internal short circuits or even fires.
- Charging to higher voltages increases energy capacity but reduces battery lifespan.
- Lithium plating and electrolyte breakdown speed up battery degradation.
- Safety risks include dendrite growth and thermal runaway, especially in automotive and electric vehicles.
Manufacturers use several safety mechanisms in lithium-ion battery design. Inside the cell, you find devices that limit current and release pressure safely. Outside, electronic circuits stop charging if the voltage or temperature gets too high. These protections help prevent failures in rechargeable batteries used in electronics, vehicles, and the automotive sector.
| 安全功能 | Function |
|---|---|
| PTC Device | Stops high current surges |
| Circuit Interrupt Device (CID) | Opens circuit if pressure rises |
| Safety Vent | Releases gas to prevent rupture |
| Electronic Protection Circuit | Cuts off charging at unsafe voltage/temperature |
You benefit from these design features every time you use lithium-ion batteries in your electronics or vehicles. The 3.7V standard voltage supports high energy density and reliable safety, making lithium-ion battery technology a top choice for modern rechargeable applications.
Industry Standards and Compatibility
Global Adoption of 3.7V
You see the 3.7V standard everywhere in the world of lithium batteries. Major manufacturers like BYD, ATL, Gotion High-Tech, and CBAK Energy Technology all use this voltage in their products. They make batteries for many applications, including consumer electronics, smart home devices, and wearable technology. The 3.7V nominal voltage is especially common in lithium polymer batteries. You find these batteries in Bluetooth headsets, GPS trackers, and medical equipment. This global adoption shows that the 3.7V standard is not just popular—it is essential for the industry.
Why do so many companies choose this voltage? The answer comes from the chemistry inside the battery. The natural electrochemical potential of lithium cobalt oxide chemistry averages around 3.7V. This voltage range, from 3.0V to 4.2V, gives you a balance between safety, performance, and energy density. Many devices and their components work best at 3.3V or 3.7V, which makes circuit design easier and reduces wasted energy. Battery Management Systems (BMS) are also designed for 3.7V cells, making engineering safer and more reliable. Using this standard helps companies lower costs, speed up innovation, and meet global safety rules.
请注意: The 3.7V standard lets you build battery packs for electric vehicles and the automotive sector by connecting cells in series. This modular approach gives you the voltage you need for larger vehicles and more demanding applications.
Device Compatibility and Design
You benefit from the 3.7V standard every time you use modern electronics. This voltage matches the needs of many portable and low-to-medium power devices. It allows stable operation without extra changes to the design. Because so many manufacturers use the same standard, you can easily find batteries for your devices. This makes repairs and upgrades simple.
When you design a device, you must consider the chemistry and voltage of the battery. A 3.7V lithium-ion battery can reach up to 4.2V when fully charged. You need to design chargers and circuits to match this range. Safety is always a top concern. You use a Battery Management System to control temperature, current, and voltage. Cell balancing prevents overcharging or deep discharging, which keeps your battery safe and long-lasting.
Here are some key design considerations for devices using 3.7V lithium batteries:
- Choose the right chemistry for your application, such as lithium polymer for flexibility or lithium iron phosphate for extra safety.
- Design the enclosure to protect the battery and user, especially in vehicles and the automotive sector.
- Add safety features like BMS and thermal management to prevent overheating.
- Follow certification and regulatory rules for safety and market approval.
You see the 3.7V standard in electric vehicles, automotive systems, and consumer electronics. This standard supports high energy density, long life, and reliable safety. It helps you enjoy powerful, safe, and efficient devices in every part of your life.
Future of Lithium Battery Voltage
New Chemistries
You will see new battery chemistries changing the way lithium batteries work in the future. Scientists are exploring options like sodium-ion, potassium-ion, magnesium-ion, and calcium-ion batteries. These new chemistries can offer higher voltage, better safety, and longer life for your devices and vehicles. Some of these batteries, such as magnesium-ion and calcium-ion, can even operate at higher voltages than current lithium-ion cells. This means you could get more energy and better performance from the same size battery.
这里有一个 table that compares the voltage of different battery chemistries:
| 电池化学 | 标称电压 (V) | Voltage Characteristics and Notes |
|---|---|---|
| 锂离子(一般) | ~3.6 – 3.7 | Standard voltage range; LiFePO₄ variant at ~3.2V; full charge ~4.2V; discharge cutoff varies by chemistry. |
| Sodium-ion | 不适用 | Comparable or potentially higher energy density than Li-ion; improved safety. |
| Potassium-ion | 不适用 | Similar to sodium-ion; improved stability and safety; voltage not explicitly detailed. |
| Magnesium-ion | Higher than Li-ion | Can operate at higher voltages than Li-ion; potential for higher energy density and safety. |
| Calcium-ion | Higher than Li-ion | Higher voltage operation; improved safety via stable SEI layers. |
| Solid-state batteries | 不适用 | Focus on electrolyte and structural advantages; voltage output not specifically detailed. |
You can see that new chemistries may bring higher voltage and better safety to your devices and vehicles. These changes could help electric vehicles go farther and last longer.
Trends in Battery Design
You will notice big changes in battery design as voltage standards rise. Electric vehicles are moving from 400V systems to 800V or higher. This shift means you need new materials for cables and connectors. These materials must handle more power and heat while keeping safety high. You will see lighter, thinner cables with better insulation and smart features. Some cables will have sensors to check temperature and performance in real time.
- New conductor materials, like aluminum alloys, help reduce weight in vehicles.
- Nanomaterials improve how well cables carry electricity and manage heat.
- Smart cables can warn you about problems before they cause damage.
You will also see more eco-friendly materials in battery systems. These changes help vehicles meet strict rules for recycling and safety. As voltage standards change, your devices and vehicles will become safer, more efficient, and ready for the future.
小贴士 Higher voltage batteries mean faster charging and better performance for electric vehicles, but you must always keep safety as your top priority.
你看 3.7V standard in lithium batteries for good reasons:
- This voltage keeps the battery stable and helps it last longer.
- Storing batteries near 3.7V reduces chemical stress and slows down wear.
- Going above or below this voltage can damage the battery.
The 3.7V standard also gives you safe, efficient, and reliable power for many devices. You benefit from built-in protections and easy compatibility across electronics. As battery technology grows, you can expect even better performance and new chemistries that build on this strong foundation.
常见问题
What does the 3.7v standard voltage mean for lithium batteries?
You see the 3.7v standard voltage as the average voltage most lithium-ion batteries deliver during normal use. This value helps you compare batteries and makes battery design easier for electronics and electric vehicles.
Why do lithium-ion batteries have high energy density?
Lithium-ion battery chemistry lets you store more energy in a small space. You get high energy density because lithium moves easily between the anode and cathode. This feature supports high performance in consumer electronics and vehicles.
How does battery design affect safety in rechargeable batteries?
Good battery design protects you from risks. Engineers add safety features like vents and electronic circuits. These parts stop overcharging and overheating. You can use lithium batteries in many applications, including the automotive sector, with confidence.
Can you use lithium batteries in all electronics and vehicles?
You find lithium batteries in many electronics and vehicles because they offer high specific energy and long life. Some applications, like the automotive sector, need special battery design for extra safety and performance.

