1,What is a Lithium-ion Battery?
Lithium-ion batteries (LIBs), as representatives of secondary batteries (rechargeable batteries), are currently the most widely used batteries. Lithium-ion batteries typically consist of positive electrodes, negative electrodes, separators, electrolytes, and casings. During cycling, they rely on the insertion and deinsertion of lithium ions between the positive and negative electrodes through the internal circuit and the back-and-forth movement of electrons in the external circuit to achieve charging and discharging. Depending on the positive electrode material, packaging, and application, lithium-ion batteries can be classified accordingly. Under long-term charge-discharge cycles, lithium-ion batteries undergo a series of side reactions, including lithium plating and the growth of solid electrolyte interface (SEI) films, which reduce the battery’s capacity. When the battery capacity decreases to 80% of the initial capacity, it is defined as the end of the battery’s service life. Their characteristics include high specific energy, long service life, high operating voltage, wide operating temperature range, no memory effect, low self-discharge, and environmental friendliness. Therefore, they are widely used in areas such as new energy vehicles, grid energy storage, and portable electronic devices.

2,Development History of Lithium-ion Batteries
In the 1950s, the U.S. Department of Defense and the National Aeronautics and Space Administration (NASA) studied high-energy-density batteries using metal fluorides as positive electrodes and lithium metal as negative electrodes. However, due to issues such as lithium dendrites, the performance was not ideal.
In the 1970s, John B. Goodenough and his research team at Exxon Mobil discovered that lithium ions could migrate rapidly in TiS2. They designed the first lithium battery with TiS2 as the positive electrode and lithium metal as the negative electrode. Although it was unstable and had low energy density, it was still used for 40 years.
In 1980, Goodenough’s team selected LiCoO2 as the positive electrode material, which increased the energy density with a high voltage of 4V, although lithium metal was still used as the negative electrode.
In 1982, researchers at the Illinois Institute of Technology, including Prabakar Prabhakar and Selman, discovered that lithium ions could be inserted into graphite, improving the safety of lithium batteries. Bell Labs successfully developed the first usable lithium-ion graphite battery.
In 1990, Sony Corporation officially commercialized lithium-ion batteries with graphite as the negative electrode and lithium-containing compounds as the positive electrode, distinguishing them from lithium batteries with metal negative electrodes, officially naming them lithium-ion batteries.
In 1996, Goodenough’s team discovered that lithium iron phosphate with an olivine structure as the positive electrode material was safer and more resistant to high temperatures and overcharging. It has become the mainstream positive electrode material and has been widely used.
Since the 21st century, with the efforts of scientists, higher capacity ternary positive electrode materials with different proportions of Ni, Co, Mn, and Al transition metal oxides have emerged successively, driving the development of lithium-ion batteries.
In 2019, the Nobel Prize in Chemistry was awarded to John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino for their outstanding contributions to the development of lithium-ion batteries.
3,Working Principle of Lithium-ion Batteries
Lithium-ion batteries, as electrochemical energy storage devices, involve the conversion of electrical energy and chemical energy. They essentially act as concentration cells, also known as “rocking chair batteries.” During charging, the applied external voltage causes lithium ions in the positive electrode to deintercalate and intercalate into the negative electrode through the electrolyte via a separator. Simultaneously, to maintain charge neutrality, electrons from the positive electrode flow through the external circuit to the negative electrode. As lithium ions continuously deintercalate from the positive electrode material and intercalate into the negative electrode, the potential of the positive electrode increases, while that of the negative electrode decreases, resulting in an increase in battery voltage (positive electrode potential minus negative electrode potential) until the charging cut-off voltage is reached.
During discharge, when the battery is connected to an external load, lithium ions deintercalate from the negative electrode due to the potential difference between the positive and negative electrodes. They then flow through the electrolyte and separator to intercalate into the positive electrode. As the potential of the negative electrode gradually increases due to lithium ion deintercalation, and the potential of the positive electrode decreases, the battery voltage decreases until the discharge cut-off voltage is reached. The working principle is illustrated in the diagram below.
Ideally, the intercalation and deintercalation of lithium ions do not affect the structure of the active material, making the reaction reversible.
Positive Electrode of Lithium-ion Battery
The positive electrode of a lithium-ion battery consists of positive electrode active material, binders, conductive agents, and current collectors. The positive electrode active material is the most crucial component, providing the lithium ions needed for battery cycling. It participates in electrochemical reactions, intercalates and deintercalates lithium ions, and conducts electrons to maintain electrical neutrality. The performance and cost of the positive electrode material significantly affect the overall performance and cost of the battery.
Positive Electrode Active Material: The output voltage and available capacity of the battery determine the energy it can store. To maximize battery performance and lifespan, positive electrode active materials must meet the following requirements:
Have a high redox potential to increase the potential difference with the negative electrode and raise the battery’s output voltage.
Can intercalate as many lithium ions as possible to determine the battery’s available capacity.
Undergo minimal structural changes during lithium ion intercalation and deintercalation to improve battery life and reliability.
Low cost, environmentally friendly.
Good chemical stability and thermal stability, no reaction with the electrolyte.
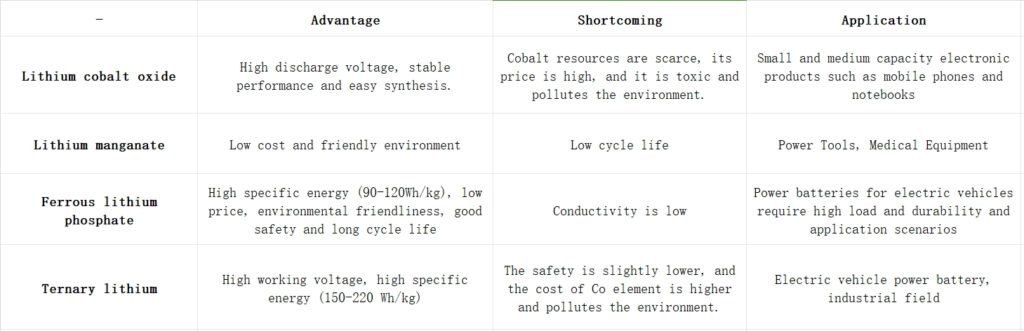
Common positive electrode materials include lithium cobalt oxide, lithium manganese oxide, lithium iron phosphate, and ternary positive electrode materials such as lithium nickel cobalt manganese oxide and lithium nickel cobalt aluminum oxide.
Negative Electrode of Lithium-ion Battery
The negative electrode of a lithium-ion battery consists of negative electrode active material, binders, conductive agents, and current collectors. Similar to the positive electrode active material, the negative electrode active material needs to participate in lithium ion intercalation and deintercalation, electron conduction, and maintain electrical neutrality. However, when the battery is first assembled, the negative electrode does not contain lithium ions. Ideal negative electrode active materials must meet the following requirements:
High capacity for lithium intercalation to increase battery capacity.
Good lithium diffusion in the negative electrode active material to withstand high-current charging and discharging.
Good conductivity to prevent electrode polarization.
Minimal structural changes during lithium intercalation and deintercalation.
Low cost, good stability, easy to manufacture, environmentally friendly.
Since Sony’s commercial production in 1990, the widely used negative electrode material has been graphite. Graphite has a complete layered crystal structure and has advantages such as low cost, high structural stability, non-toxicity, good conductivity, and mechanical properties, making it suitable for many applications.
However, as the industry continues to demand higher battery performance, graphite-based negative electrode lithium-ion batteries have disadvantages when used in high-power devices, such as low power and poor safety. In the fields of research and high-tech products, a new generation of negative electrode materials for lithium-ion batteries has emerged to meet the demand for high-power application batteries. In 2005, Sony introduced a technically safe and low-cost amorphous/nanocrystalline composite negative electrode material Sn/Co/C, with a stable reversible capacity of up to 450 mA·h/g, driving the rapid development of a series of new negative electrode materials (silicon-based materials, titanium-based materials, metal oxides, and sulfides).
Lithium-Ion Battery Separator
The separator inside lithium-ion batteries typically serves two purposes:
It electrically isolates the positive and negative electrodes of the battery to prevent internal short circuits.
The ion channels within the separator allow ions in the electrolyte to pass freely, ensuring the formation of a normal electric current loop inside the battery during charge and discharge cycles.
Common separator materials are generally polyolefin-based resins. For example, the Celgard2400 separator is a three-layer microporous membrane of PP (polypropylene)/PE (polyethylene)/PP.
Lithium-Ion Battery Electrolyte
The role of the electrolyte in lithium-ion batteries is to facilitate ion transfer between the positive and negative electrodes during the electrochemical reaction. Due to the high reactivity of lithium ions with the negative electrode, the electrolyte in lithium-ion batteries uses non-aqueous, non-protonic organic solvents as ion carriers. The electrolyte needs to have sufficient electrical conductivity, thermal stability, chemical stability, and film-forming characteristics, while also meeting low cost, ease of preparation, and environmental friendliness.
The commonly used electrolyte for lithium-ion batteries is a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) as solvents.
Lithium-Ion Battery Casing
The main function of the casing is to act as the battery’s container, protecting the internal materials of the battery. Since lithium-ion batteries are often subjected to external pressure during actual use, the casing needs to increase the mechanical strength of the battery to prevent deformation of the internal materials (especially the separator, which has the lowest stiffness), thereby affecting the safety and lifespan of the battery. High-quality aluminum is commonly selected as the material for lithium-ion battery casings.
4.Advantages and Disadvantages of Lithium-Ion Batteries
Advantages:
High operating voltage: The operating voltage of lithium-ion batteries is as high as 3.6V, which is three times that of nickel-chromium and nickel-metal hydride batteries, and twice that of lead-acid batteries.
High energy density: Due to the high operating voltage and low lithium density, lithium-ion batteries have a high mass energy density (200Wh/kg) and volumetric energy density (350Wh/L), which is three times that of lead-acid batteries (50-70Wh/kg).
Low self-discharge rate: The capacity drop caused by spontaneous reactions inside lithium-ion batteries when no external load is applied is relatively low.
Long cycle life: Lithium-ion batteries used in practical applications can be charged and discharged more than 1000 times.
No memory effect: The battery capacity does not decrease due to incomplete discharge before charging.
Wide operating temperature range: -20°C to 60°C.
Disadvantages:
High cost: Lithium-ion batteries are 3-4 times more expensive than lead-acid batteries of the same capacity.
Poor low-temperature performance: The use of organic solvents as electrolytes limits the low-temperature performance, and low-temperature charging can cause additional overpotential at the negative electrode, leading to lithium plating, affecting battery life and safety.
Poor overcharging performance: Exceeding a certain charging voltage can cause decomposition of the electrolyte and electrode active materials due to poor thermal stability, releasing a large amount of heat, affecting battery safety.
Poor safety: High energy density can lead to the rapid release of a large amount of energy in the event of a failure, making it prone to explosions and other severe safety accidents.
5.Classification of Lithium-Ion Batteries
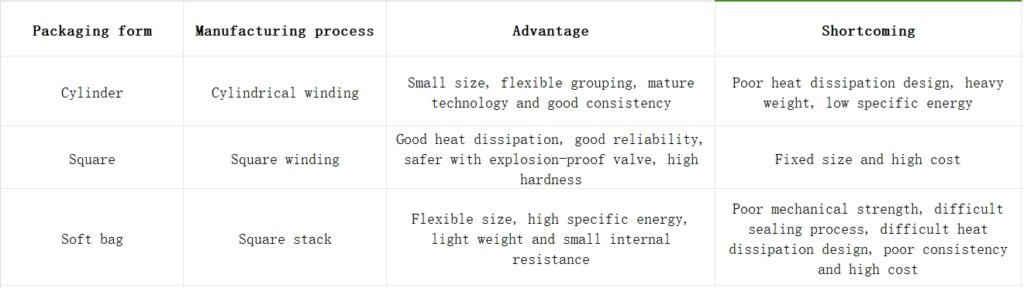
Classification by packaging form: Lithium-ion batteries are classified into cylindrical, prismatic, and pouch cells based on their packaging form. The battery packaging form is closely related to the manufacturing method and product performance.
Classification by positive electrode material: Lithium-ion batteries are classified into lithium cobalt oxide, lithium manganese oxide, lithium iron phosphate, and ternary lithium-ion batteries based on different positive electrode materials. The advantages and disadvantages of different positive electrode materials are compared.
Classification by application: Lithium-ion batteries are classified into energy-type, power-type, and power-type batteries based on their application scenarios and requirements for power and energy density.
6.Aging Mechanism of Lithium-Ion Batteries
The aging of lithium-ion batteries is the result of the combined effects of cycle aging and calendar aging (long-term storage), making it a highly complex and long-term process. Various types of internal physical and chemical processes contribute to battery aging, including the formation of the Solid Electrolyte Interphase (SEI) membrane and lithium plating.
SEI Growth in Lithium-Ion Batteries
During the initial charging (formation) process of the battery, about 10% of the lithium is consumed at the interface between the negative electrode and the separator to form the initial SEI membrane. Throughout the battery’s entire lifecycle, the SEI membrane undergoes a cyclic process of formation, growth, decomposition, and regeneration. The SEI membrane is a product of various reactions between lithium ions and components in the electrolyte, leading to a reduction in reversible lithium and consequently reducing the battery’s capacity.
Lithium Plating in Lithium-Ion Batteries
Under conditions of low temperature, high rates, and overcharging, when the lithium potential at the negative electrode drops below 0V, some lithium ions continue to embed into the graphite electrode while others precipitate out on the surface of the graphite electrode in the form of metallic lithium, a phenomenon known as lithium plating. This not only reduces the amount of reversible lithium but also leads to the growth of dendrites, which may penetrate the separator, causing severe safety hazards such as internal short circuits.
7.Safety Faults of Lithium-Ion Batteries
Lithium-ion batteries, as high-energy storage devices, inherently pose certain risks due to defects in materials and structural design during the manufacturing process. The working environment of batteries is also highly complex. Besides frequent collisions, vibrations, and impacts, the battery system also releases a significant amount of heat during operation, all of which can lead to safety hazards. Failure modes of lithium-ion battery safety include internal short circuits, external short circuits, overcharging/overdischarging, and others.
Internal Short Circuit of Lithium-Ion Batteries
An internal short circuit of the battery refers to the phenomenon where the positive and negative electrode materials form an electrical connection inside the battery. This leads to discharge due to the potential difference and is accompanied by a significant release of heat. When a battery experiences an internal short circuit, a large amount of energy is rapidly released within a short period, causing the battery to heat up rapidly, and in extreme cases, leading to battery explosions.
External Short Circuit of Lithium-Ion Batteries
Unlike internal short circuits, an external short circuit of the battery occurs when the positive and negative electrodes directly form an electrical connection outside the battery. The battery discharges through minimal resistance, causing the chemical energy stored inside the battery to dissipate as heat energy. This results in rapid heating of the battery, although the peak temperature is lower compared to internal short circuits. External short circuits are typically caused by deformation due to collisions, water immersion, and connection failures in the battery system.
Overcharging/Overdischarging of Lithium-Ion Batteries
Overcharging (overdischarging) refers to the continued charging (discharging) of a battery after it is fully charged (discharged). Both rapid charging (discharging) and high-rate charging (discharging) in the later stages can lead to overcharging (overdischarging). Mild overcharging (overdischarging) may only cause a slight decrease in the battery’s available capacity, but prolonged overcharging (overdischarging) can affect battery safety.

8.Safety Considerations for Lithium-Ion Batteries
Lithium-ion batteries do not pose a hazard to humans or the environment during normal use. However, improper handling during disposal, such as not properly discharging, dismantling, crushing, and sorting, can lead to environmental harm, affecting surrounding organisms and humans.
Materials such as cobalt in the active materials of lithium-ion batteries, lithium hexafluorophosphate in the electrolyte, and polyethylene in the separator can cause organic pollution to the environment. During disposal, these materials need to be discharged to an empty state first. Then, plastic and iron casings should be dismantled and recycled. The electrode materials should undergo alkali and acid leaching, followed by extraction. Electrolytes, electrode liquids, and some conversion and hydrolysis products (lithium hexafluorophosphate, hydrogen fluoride, methanol, formic acid, etc.) need to be sent to qualified facilities for proper disposal and cannot be discarded casually. Recycling key electrode materials not only reduces environmental pollution but also reduces dependence on resources such as lithium and cobalt, carrying significant social and economic significance.
Lithium-ion batteries typically reach the end of their lifespan when their capacity decreases to 80%. However, directly scrapping them would result in significant resource wastage. They can be utilized through cascade utilization in multiple application scenarios. After leaving the factory, batteries can first be used in electric vehicles. After one cycle of life ends, they can be applied in grid energy storage systems with low power requirements. This approach not only extends the battery’s lifespan but also reduces its cost.
9.Applications of Lithium-Ion Batteries
Automotive Sector
In response to the national dual carbon policy and the need to address energy shortages and environmental pollution, the automotive industry needs to transition from fuel vehicles to electric vehicles. Lithium-ion batteries, with their high energy density, high operating voltage, and long cycle life, have been widely used in both electric cars and electric buses, especially lithium iron phosphate batteries and ternary system batteries.
Grid Energy Storage Sector
To further reduce dependence on non-renewable energy sources (coal, oil, natural gas) and promote renewable energy sources (wind, solar, etc.), renewable energy generation has received widespread attention. However, renewable energy generation has certain fluctuations and intermittencies, making direct transmission to the grid impractical and causing grid instability. Therefore, energy storage batteries are needed to store some of the converted electrical energy. They can supply energy to the grid during peak load periods, thus achieving grid frequency regulation and peak shaving. Energy storage batteries do not have a direct requirement for energy density, but different energy storage scenarios (frequency regulation, peak shaving) have requirements for battery power density. Lithium-ion batteries with long lifespans and high energy conversion efficiency can fulfill these requirements.
Portable Electronic Devices Sector
In recent years, with the rise of technologies such as the Internet, the Internet of Things (IoT), and big data, there has been an increasing number of electronic devices, making life more convenient for residents. Lithium-ion batteries are found in various electronic devices such as smartphones, MP3 players, MP4 players, cameras, remote controls, children’s toys, etc.
10.Trends in the Development of Lithium-Ion Batteries
China, as the most active region in the global development of lithium-ion batteries, has seen the market size of lithium-ion batteries grow year by year. New energy vehicles are the biggest engine driving the rapid growth of lithium-ion batteries. The demand for lithium-ion batteries in the energy storage market is also expected to accelerate. In addition to meeting domestic demand, lithium-ion batteries will also be exported in large quantities to Europe, America, Australia, Southeast Asia, and other regions.
Countries worldwide attach great importance to the development of the lithium-ion battery industry. Japanese company Panasonic and South Korean company LG Energy Solutions, as the two major lithium-ion battery giants second only to China’s CATL, aim to occupy a higher market share. In May 2018, the European Commission released the “Battery Strategy Action Plan,” proposing a long-term research plan for battery technology over the next ten years called “BATTERY 2030+.” Its vision is to invent the batteries of the future, creating disruptive technology and competitive advantages across the entire value chain for European industry. BATTERY 2030+ aims to pursue batteries with ultra-high performance, reliability, safety, sustainability, and affordability by using interdisciplinary research methods and advanced technologies such as artificial intelligence, robotics, sensors, and smart systems.
According to the “Energy-Saving and New Energy Vehicle Technology Roadmap” released by the Ministry of Industry and Information Technology, the energy density target for power batteries in 2030 is 500Wh/kg. Currently, the highest energy density of NCM ternary batteries is only 250-300Wh/kg. Increasing the nickel content in ternary systems is expected to further improve energy density, but it may also affect safety. How to increase energy density without compromising safety is an important issue that needs to be addressed. Poor battery performance in cold weather has always been a pain point for lithium-ion batteries. Developing all-weather batteries that can adapt to low temperatures is also a future direction for lithium-ion battery development. Additionally, efforts should be made to improve cycle life and reduce costs, which are all future research hotspots.

