
Ternary lithium batteries lead the global market in 2025, especially for electric vehicles, due to their higher energy density and longer lifespan. LCO Battery Technology remains a top choice for compact electronics where high power in a small space is critical. Users should select a battery type based on their main need: ternary lithium batteries offer advantages for vehicles, while LCO batteries deliver reliable performance in portable electronics. The right match ensures safety, value, and long-term satisfaction.
Overview
LCO Battery Technology
LCO battery technology stands as one of the earliest and most widely used forms of lithium ion batteries. The core of an LCO battery uses lithium cobalt oxide as its cathode material. This chemical composition of lithium-ion batteries gives LCO cells a high energy density and compact size. Engineers often choose LCO battery technology for portable electronics. Devices like smartphones, laptops, tablets, and cameras rely on the stable performance and small footprint of LCO battery cells.
The main advantage of LCO battery technology lies in its ability to deliver high power output in a small package. The lithium cobalt oxide cathode provides a layered structure, which supports fast charging and long cycle life. However, LCO battery cells face challenges with thermal stability and safety. Overcharging or overheating can lead to safety risks. The cost and limited supply of cobalt also affect the long-term use of LCO battery technology.
Note: LCO battery technology remains the preferred choice for medical devices and aerospace applications where reliability and energy density matter most.
Ternary Lithium Batteries
Ternary lithium batteries use a mixed metal oxide cathode. This cathode blends nickel, cobalt, and manganese (NCM) or nickel, cobalt, and aluminum (NCA). The chemical composition of lithium-ion batteries in ternary cells allows for higher specific capacity and improved cost efficiency. Ternary lithium batteries have become the standard for electric vehicles and energy storage systems.
The ternary cathode structure offers better thermal stability and a wider operating temperature range than LCO battery cells. Nickel increases capacity, cobalt improves rate performance, and manganese or aluminum adds stability. Ternary lithium batteries support longer cycle life and higher output power, making them ideal for electric vehicles and high-performance e-bikes.
| Аспект | LCO (Lithium Cobalt Oxide) Cathode | Ternary Lithium Cathodes (NCM, NCA) |
|---|---|---|
| Primary Composition | Lithium cobalt oxide (LiCoO2) | Mixed metal oxides: nickel, cobalt, manganese (NCM) or nickel, cobalt, aluminum (NCA) |
| Crystal Structure | Layered structure | α-NaFeO2 type layered structure |
| Role of Elements | Cobalt is the main active element | Nickel determines capacity; cobalt improves rate performance; manganese stabilizes lattice; aluminum enhances stability (NCA) |
| Преимущества | High working voltage, good cycle performance | Higher specific capacity, better cost efficiency, improved stability with doping and co-doping strategies |
Ternary lithium batteries now dominate the market for electric vehicles due to their balance of performance, safety, and cost. In contrast, LCO battery technology continues to serve best in compact electronics and specialized fields.
Ключевые различия
Chemical Structure
Lithium cobalt oxide and ternary lithium batteries show clear differences in their chemical structure. LCO battery technology uses a cathode made almost entirely of cobalt oxide layers. These layers create a strong and stable structure, which helps the battery deliver high energy density. In LCO battery cells, lithium ions move between the anode and the cobalt oxide cathode during charging and discharging. This simple structure makes lco battery technology reliable for portable electronics.
Ternary lithium batteries, including NMC battery types, use a more complex cathode. The cathode blends three metals: nickel, cobalt, and manganese or aluminum. This combination forms a layered structure called the α-NaFeO2 type. The metals share oxygen atoms and can switch between different oxidation states. This design allows ternary lithium batteries to store more energy and handle higher currents. The lithium ions in ternary cathodes have weaker bonds to oxygen, which increases capacity but can also lead to structural changes during use. Nickel migration and phase transitions sometimes occur, but doping with elements like aluminum or magnesium can improve stability.
Note: The mixed metal structure in ternary cathodes gives them higher capacity and better stability than pure lithium cobalt oxide cathodes.
Lithium Cobalt Oxide vs. Ternary Cathodes
The main difference between lithium cobalt oxide and ternary cathodes lies in their composition and performance. LCO battery technology relies on cobalt as the main active metal. This gives the battery high specific energy but limits its stability and lifespan. Ternary lithium batteries, such as lithium nickel manganese cobalt oxide (NMC battery), use a mix of metals. This mix improves capacity, cycle life, and safety.
| Характеристика | Оксид кобальта лития (LCO) | Ternary Cathodes (NMC, NCA) |
|---|---|---|
| Main Metals | Cobalt | Nickel, cobalt, manganese/aluminum |
| Structure | Layered cobalt oxide | Layered mixed metal oxide |
| Плотность энергии | Высокий | Выше |
| Stability | Moderate | Improved with doping |
| Typical Use | Electronics | EVs, energy storage |
Ternary lithium batteries benefit from advanced microstructural designs. For example, concentration-gradient NMC battery cathodes show better lithium-ion movement and higher capacity after many cycles. Doping and careful metal ratios help ternary cathodes resist thermal runaway and maintain performance. In contrast, lithium cobalt oxide cathodes offer simplicity and reliability but cannot match the advanced features of ternary designs. Both types remain important in the lithium ion battery market, but ternary lithium batteries lead in applications that demand high performance and long life.
Performance
Energy Density and Output Power
LCO battery technology and ternary lithium batteries both deliver impressive performance in modern devices. However, their energy density and output power differ in important ways. LCO batteries stand out for their high volumetric energy density. This means they can store a large amount of energy in a small space. Engineers often select LCO batteries for smartphones and tablets because these devices require high energy density and compact size. The lithium cobalt oxide cathode in LCO batteries supports this high volumetric energy density, making them ideal for high-power applications where space is limited.
Ternary lithium batteries, such as NMC and NCA types, also offer high energy density. Their mixed metal cathode structure allows for even higher volumetric energy density compared to LCO batteries in some cases. Nickel in the cathode increases the energy density and output power, while manganese or aluminum adds stability. This combination makes ternary lithium batteries the preferred choice for electric vehicles and other high-power applications. These batteries provide strong performance characteristics, including rapid acceleration and long driving range in EVs.
Tip: Devices that demand high energy density and high output power, such as drones and power tools, benefit from ternary lithium batteries. LCO batteries remain the top pick for compact electronics that need high volumetric energy density.
Both battery types use lithium ion movement to deliver energy. However, ternary lithium batteries often outperform LCO batteries in high-power applications due to their advanced cathode design. This design supports higher output power and better thermal management, which improves overall performance.
Цикл жизни
Cycle life measures how many times a battery can charge and discharge before its capacity drops to 70% of its original value. This factor plays a key role in the long-term performance of lithium ion batteries. Under standard conditions, both LCO batteries and ternary lithium batteries offer similar cycle life. Most LCO batteries reach about 800 cycles, while ternary lithium batteries also achieve around 800 cycles under normal use. Manufacturers often claim more than 500 cycles for ternary lithium batteries, but real-world use in battery packs can reduce this number to about 400 cycles.
| Тип батареи | Typical Cycle Life (Standard Conditions) | Примечания |
|---|---|---|
| Оксид кобальта лития (LCO) | ~800 cycles | Treated as part of ternary lithium batteries; medium cycle life among commercial types |
| Ternary Lithium Batteries | ~800 cycles | Manufacturer specs promise >500 cycles; pack assembly reduces to ~400 cycles |
| Железофосфат лития | ~2000 cycles | Longer cycle life than ternary/LCO |
| Lithium Titanate | ~10,000 cycles | Highest among common lithium battery types |
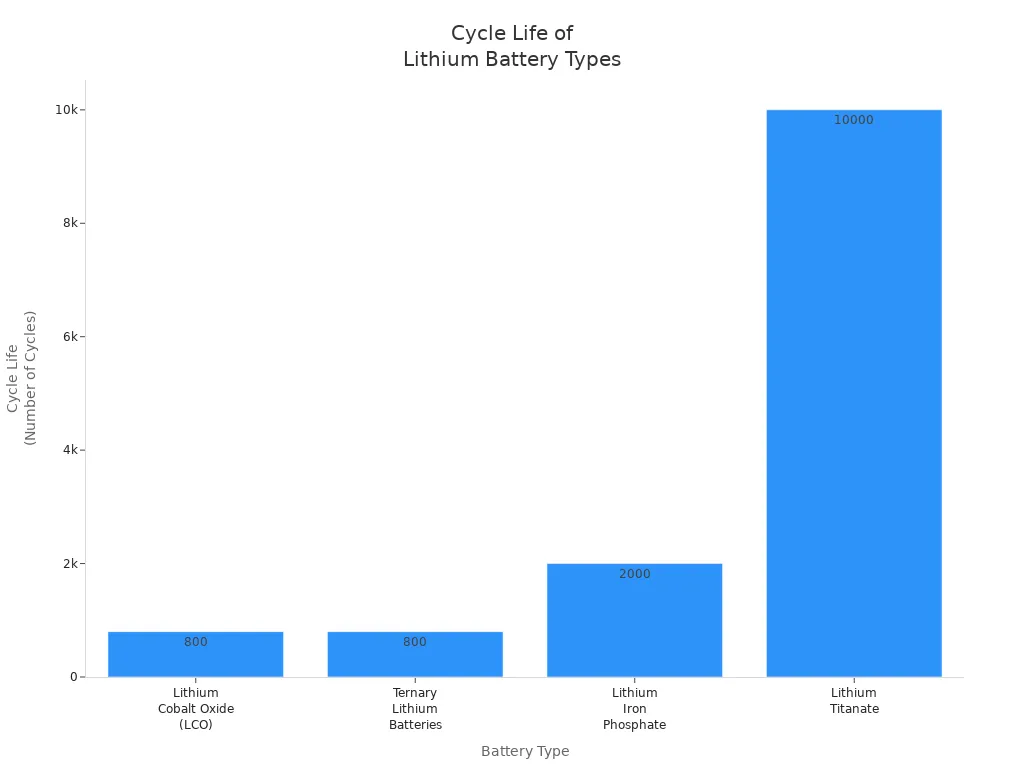
Users can extend the cycle life of both LCO and ternary lithium batteries by charging them within a state of charge window of 10% to 90%. Avoiding deep discharges and high temperatures also helps. In some cases, careful use can push the cycle life to over 1,000 cycles. However, high discharge rates or extreme heat can reduce cycle life to less than 200 cycles.
Ternary lithium batteries often show better performance in demanding environments. Their advanced cathode structure resists degradation during repeated cycling. LCO batteries, while reliable, may lose capacity faster under stress. For high-power applications that require frequent charging and discharging, ternary lithium batteries provide a performance advantage.
Note: Cycle life depends on many factors, including temperature, charge rate, and depth of discharge. Users should follow manufacturer guidelines to maximize battery performance and lifespan.
Thermal Stability and Safety
Особенности безопасности
Manufacturers design both lithium cobalt oxide and ternary lithium batteries with safety in mind. These batteries include several built-in features that help prevent accidents and extend their lifespan. The following table highlights the main safety features found in modern lithium batteries:
| Safety Feature / Parameter | Description / Value |
|---|---|
| Overcharge Protection Voltage | 4.325 ± 0.025 V per series |
| Over-discharge Protection Voltage | 2.5 ± 0.05 V |
| Working Temperature Range | -10 to 60 °C |
| Thermal Runaway Температура | 250 to 300 °C |
| Protection Plate Functions | Controls overcharge, over-discharge, short circuit, over-temperature, over-current |
| Safety Instructions | Avoid hitting or heating the battery; follow proper charging and storage guidelines |
| Charging Guidelines | Use supporting charger; avoid over-discharge; do not overcharge or leave on charger too long |
| Storage Guidelines | Store in dry, cool place; avoid contact with metal objects; avoid physical damage |
These features work together to improve thermal stability and safety. Protection plates monitor the battery’s voltage and temperature. They stop charging if the battery gets too hot or reaches unsafe voltage levels. This helps prevent overcharging, which can lead to dangerous situations. Manufacturers also provide clear instructions for charging and storage. Users should always follow these guidelines to keep the battery safe.
Ternary lithium batteries often show better thermal stability than older designs. Their mixed metal cathode structure resists overheating and supports a wider temperature range. This makes them a strong choice for electric vehicles and energy storage systems. Lithium cobalt oxide batteries, on the other hand, face unique safety challenges. When overcharged, the material can change phase and release oxygen. This process can cause cobalt to dissolve into the electrolyte, which reduces stability and increases the risk of failure. For this reason, lithium cobalt oxide batteries depend heavily on careful charging and strict management of operating conditions.
Tip: Always use the charger recommended by the manufacturer. Never leave a lithium battery charging overnight or in direct sunlight.
Risk Factors
Lithium batteries offer high energy density, but this advantage comes with risks. Both lithium cobalt oxide and ternary lithium batteries contain flammable electrolytes. If the battery gets too hot, it can enter thermal runaway. This is a chain reaction that causes the battery to overheat, catch fire, or even explode. Thermal runaway can start from overcharging, physical damage, or internal short circuits.
Some of the most common risk factors include:
- Thermal runaway remains a critical risk. Overheating can trigger a chain reaction, leading to fires or explosions. Internal short circuits, overcharging, physical damage, or excessive discharge often cause this problem. In 2023, New York City reported 92 lithium-ion battery fires, resulting in 64 injuries and 9 deaths.
- Manufacturing defects sometimes introduce contamination or improper assembly. These issues can cause internal short circuits, which may lead to overheating, fires, or explosions. One major tablet recall happened because of microscopic metal particles inside the cells.
- Design flaws, such as poor thermal management or lack of protective circuitry, increase the risk of thermal runaway. This is especially true for large batteries in electric vehicles and energy storage systems.
- Supply chain vulnerabilities affect the availability and safety of lithium batteries. Most lithium and cobalt come from a few countries, mainly China. Political pressures can disrupt supply and impact safety regulations.
- Lithium-ion battery incidents can happen in many settings. Consumer devices, electric vehicles, transportation, and industrial facilities all face safety, operational, and insurance challenges from battery failures.
Ternary lithium batteries usually provide better thermal stability than lithium cobalt oxide batteries. Their advanced cathode design helps resist overheating and supports safer operation under stress. However, all lithium batteries require careful handling and proper management to reduce risks. Users should avoid dropping, crushing, or exposing batteries to extreme temperatures. Following safety instructions and using certified products can help prevent accidents.
Note: Understanding the risks and following best practices ensures better thermal stability and safety for all lithium battery users.
Стоимость и доступность
Material Costs
Material costs play a major role in the price of both LCO and ternary lithium batteries. Cobalt stands out as one of the most expensive minerals in the battery industry. Its price often changes quickly because of supply chain disruptions and geopolitical issues, especially in countries like the Democratic Republic of Congo. When cobalt prices rise, the cost of LCO batteries increases sharply. Ternary lithium batteries also depend on cobalt for stability and performance. Even though manufacturers try to reduce cobalt content, they cannot remove it completely without losing battery quality.
Nickel and manganese also affect the cost structure. Nickel boosts energy density but can make the battery less stable if used in high amounts. Its price can swing, which impacts the final cost of ternary lithium batteries. Manganese, on the other hand, is more abundant and less expensive. It helps lower the overall cost of the cathode, but too much manganese can reduce battery capacity.
| Element | Role in Battery Cathode | Impact on Cost and Performance |
|---|---|---|
| Nickel | Increases energy density and capacity | Price swings affect cost; high content may reduce stability |
| Cobalt | Stabilizes structure, improves cycle life and safety | High cost; price changes impact battery price |
| Manganese | Enhances thermal stability, reduces cost | Lower cost; less impact from price changes |
Note: The price of lithium itself also matters, but cobalt and nickel have the biggest impact on battery cost.
Market Trends
The market for lithium batteries continues to grow quickly. Manufacturers focus on improving the mix of nickel, cobalt, and manganese to boost energy density and make batteries last longer. Demand for ternary lithium batteries rises as more people buy electric vehicles and portable electronics. Government policies that support clean energy and offer subsidies for electric vehicles help drive this growth.
- The global LCO battery market could reach $32 billion by 2025, with strong demand from smartphones and AR devices.
- LCO batteries remain popular for their compact size and high energy density, especially in mobile devices.
- Ternary lithium batteries see rapid growth because of electric vehicle adoption and clean energy initiatives.
- Supply chain challenges, such as raw material shortages and price swings, create risks for both battery types.
- New recycling methods and sustainable sourcing help reduce dependence on raw materials and support environmental goals.
China leads the global supply chain for lithium batteries, thanks to its large manufacturing base and government support. Emerging markets in South America and Eastern Europe are gaining ground because of resource availability and investment. The automotive sector remains the biggest driver for ternary lithium battery demand, especially as more countries push for electric mobility.
Tip: Companies invest heavily in research and development to improve battery performance, safety, and cost. This trend is likely to continue through 2025.
Приложения

LCO Battery in Electronics
LCO battery technology remains a top choice for portable electronics. Engineers select this battery for devices that need high energy in a small space. Smartphones, tablets, and cameras all rely on LCO battery cells. These devices benefit from the high energy density and stable voltage that LCO battery technology provides. Users expect their portable electronics to last all day without frequent charging. LCO battery cells help meet this demand.
Manufacturers also use LCO battery technology in medical devices and some aerospace equipment. These fields require reliable power and compact size. The stable performance of LCO battery cells supports sensitive electronics, such as heart monitors and navigation tools. In portable electronics, safety and size matter most. LCO battery cells deliver both, making them a trusted solution.
Note: LCO battery technology supports the slim designs and lightweight features that consumers want in modern portable electronics.
Ternary Lithium in EVs
Ternary lithium batteries have transformed the electric vehicle industry. Automakers choose these batteries for their high energy density and strong power output. The cathode in ternary lithium batteries combines nickel, cobalt, and manganese or aluminum. This mix gives the battery more energy than LCO battery cells. Drivers experience longer ranges in electric vehicles because of this высокая плотность энергии.
Ternary lithium batteries also support faster charging and better acceleration. The improved power density allows electric vehicles to charge quickly and respond faster on the road. LCO battery cells, while powerful, cannot match the balance of range, speed, and safety that ternary lithium batteries offer for EVs. Cost and safety concerns limit the use of LCO battery technology in large vehicles.
- Ternary lithium batteries provide:
- Longer driving range for electric vehicles
- Faster charging times
- Better acceleration and power delivery
Automakers continue to invest in ternary lithium batteries to meet the growing demand for electric vehicles. This battery technology supports the shift toward cleaner transportation and helps reduce emissions worldwide.
Future Outlook
Advancements
Recent years have seen major progress in both LCO and ternary lithium battery technology. Researchers have focused on new electrode materials and better manufacturing methods. These changes increase energy density and make batteries last longer. Companies now produce batteries that cost less and work more efficiently. Some LCO batteries can improve electric vehicle range by up to 50% compared to older lithium-ion batteries.
Ternary lithium batteries use a mix of nickel, cobalt, and manganese. This mix gives them higher energy density and a longer lifespan. These batteries help electric vehicles go farther and charge faster. The market for ternary lithium batteries is growing quickly. Experts expect it to reach $8.6 billion by 2033. Ongoing research also improves battery safety and recycling. New recycling methods use artificial intelligence to make the process faster and more efficient. These advancements help reduce waste and support sustainability goals.
Note: Better battery performance and recycling technology will help meet the needs of electric vehicles, consumer electronics, and renewable energy storage.
2025 Trends
By 2025, the battery industry will face new challenges and opportunities. Governments around the world are making strict rules to protect the environment. These rules push companies to use cleaner materials and recycle more batteries. Many countries now offer incentives for electric vehicles and clean energy. This support helps the lithium battery market grow.
Environmental concerns remain important. Mining for cobalt and nickel can harm the land and water. Recycling batteries reduces this impact, but the process uses a lot of energy. Using cleaner power for recycling can lower emissions. Companies are also working on batteries that use less cobalt or none at all. These changes make batteries safer and better for the planet.
| Trend | Impact on Battery Industry |
|---|---|
| Stricter regulations | Cleaner production and more recycling |
| EV incentives | Higher demand for lithium batteries |
| New recycling tech | Lower environmental impact |
| Low-cobalt designs | Safer and more sustainable batteries |
Tip: Companies that invest in green technology and follow new rules will lead the battery market in 2025.
Choosing the Right Battery
Decision Factors
Selecting the right battery for a specific application requires careful consideration of several key factors. Energy density stands out as a primary concern. Devices that need compact, high-capacity power sources, such as smartphones or medical equipment, often benefit from lithium cobalt oxide technology. Ternary lithium batteries, which use a blend of nickel, cobalt, and manganese, provide a balance between высокая плотность энергии and cost reduction. However, these chemistries may have lower thermal stability and safety compared to alternatives like lithium iron phosphate.
Cost also plays a significant role. The price of cobalt can fluctuate, making lithium cobalt oxide batteries more expensive and less predictable in terms of long-term investment. Ternary lithium batteries offer more stable pricing because they use a mix of metals, which helps reduce reliance on any single raw material.
Safety and cycle life are equally important. Both lithium cobalt oxide and ternary lithium batteries require advanced thermal management to prevent overheating and ensure safe operation. Applications that demand longer cycle life or operate in high-temperature environments may need to consider other chemistries, but for high energy density and moderate safety needs, these two types remain popular.
The following table summarizes the main decision factors:
| Decision Factor | LCO Batteries (Lithium Cobalt Oxide) | Ternary Lithium Batteries (e.g., NMC) | Notes on Application Use |
|---|---|---|---|
| Плотность энергии | High energy density, suitable for compact, high-capacity needs | High energy density, often higher power ratings than LFP | Both favored for shorter duration, high energy applications like EVs and power tools |
| Цикл жизни | Moderate cycle life | Moderate cycle life, generally less stable than LFP | Not ideal for very long cycle life applications |
| Термическая стабильность | Lower thermal stability, riskier due to cobalt content | Reduced stability compared to LFP, lower thermal runaway temperature | Safety concerns higher than LFP; requires careful thermal management |
| Безопасность | Less safe, higher risk of thermal runaway | Less safe than LFP, but better than LCO in some cases | Safety is a critical factor for extended or abusive use |
| Стоимость | Higher cost and price volatility due to cobalt | Lower cost per watt-hour than LCO, but still influenced by nickel and cobalt prices | Cost sensitive applications may prefer ternary lithium over LCO |
| Intended Usage | Best for high energy density needs over shorter durations | Suitable for high power and energy density, but better for shorter use periods | Longer duration or multi-shift use favors LFP over LCO/NMC |
| Operating Temperature | Less tolerant to elevated temperatures | Less stable at high temperatures compared to LFP | Applications with elevated operating temperatures favor LFP |
Tip: Always match the battery’s strengths to the device’s main requirements. Consider energy density, safety, cost, and expected usage duration before making a final choice.
Recent studies show that LCO and ternary lithium batteries share a layered cathode structure but differ in performance and safety. The table below highlights these differences:
| Характеристика | LCO (Lithium Cobalt Oxide) | NMC (Nickel Manganese Cobalt Oxide) |
|---|---|---|
| Плотность энергии | 150-220 Wh/kg | 150-300 Wh/kg |
| Цикл жизни | 500-1000 cycles | Thousands of cycles |
| Безопасность | Higher risk | Improved thermal stability |
For 2025, ternary lithium batteries lead in electric vehicles, while LCO batteries remain best for portable electronics.
Users should match battery choice to their needs and watch for new lithium technology trends.
ЧАСТО ЗАДАВАЕМЫЕ ВОПРОСЫ
What makes ternary lithium batteries better for electric vehicles?
Ternary lithium batteries offer higher energy density and longer driving range. Automakers prefer them for electric vehicles because they support fast charging and deliver strong power output. These features help electric vehicles perform better on the road.
Are LCO batteries safe for everyday electronics?
LCO batteries remain safe for daily use in electronics when users follow manufacturer guidelines. Built-in protection circuits help prevent overcharging and overheating. Users should avoid exposing devices to extreme heat or physical damage.
How do material costs affect battery prices in 2025?
Material costs, especially for cobalt and nickel, influence battery prices. Ternary lithium batteries use less cobalt than LCO batteries, which helps control costs. Price swings in raw materials can still impact the final cost for consumers.
Can users recycle LCO and ternary lithium batteries?
Yes, users can recycle both battery types. Specialized recycling centers recover valuable metals like cobalt and nickel. Recycling helps reduce environmental impact and supports a more sustainable battery industry.
Which battery lasts longer: LCO or ternary lithium?
Ternary lithium batteries usually last longer in high-power applications. Their advanced cathode design resists wear during repeated charging and discharging. LCO batteries provide reliable performance but may lose capacity faster under heavy use.

