
Battery safety is a major concern for consumers. Many fires involving a lithium-ion battery stem from a dangerous process called thermal runaway. This issue is especially common in ternary lithium batteries. In fact, one-third of electric vehicle accidents are linked to this overheating during charging. The unstable chemistry of ternary lithium batteries makes them vulnerable. LiLO battery technology offers a safer path. Its stable chemical structure resists overheating. This core difference makes the LiLO battery a more secure lithium battery, unlike volatile ternary lithium batteries. The instability of ternary lithium batteries remains a key problem that LiLO solves.
The Foundation of LiLO Safety
The safety of LiLO battery technology is not an accident. It comes from the fundamental science of its internal structure. Unlike other lithium batteries that rely on volatile materials, the LiLO battery uses a chemistry that is inherently stable and resistant to the conditions that cause fires. This section explores the core reasons why this battery is a safer choice.
Stable Phosphate Chemistry
The secret to LiLO’s safety lies in its chemical makeup: Lithium Iron Phosphate (LiFePO₄). The atoms in this material are held together by incredibly strong bonds. Specifically, the bond between the phosphorus and oxygen atoms (P-O) in the phosphate structure is much stronger than the metal-oxygen bonds found in ternary lithium batteries like NCM (Nickel Cobalt Manganese).
This powerful P-O bond acts like a lock, holding oxygen atoms tightly in place. Even under stress, such as overheating or physical damage, the structure resists breaking down and releasing oxygen. Oxygen is a key ingredient for fire, so preventing its release is critical. Ternary lithium chemistries, in contrast, can release oxygen when they overheat, creating a dangerous situation where the battery itself provides the fuel for a fire. The stable olivine crystal structure of LiLO also experiences very little change in size during charging and discharging, reducing internal stress on the battery over its lifetime.
High Thermal Stability
A battery’s ability to handle heat is a direct measure of its safety. Thermal runaway is an uncontrolled chain reaction where a battery gets hotter and hotter, often leading to fire. The temperature at which this process begins is a crucial safety metric.
LiLO batteries excel in this area. Their stable chemistry gives them a much higher tolerance for heat. In laboratory tests comparing different battery types, the point of thermal runaway was often not even detected for the LiLO battery. This demonstrates its exceptional resistance to overheating.
Let’s look at the data directly:
| Type de batterie | Thermal Runaway Onset Temperature (°C) |
|---|---|
| NCM811 | 147.35 °C (297 °F) |
| ANC | 165.44 °C (330 °F) |
| LCO | 180.16 °C (356 °F) |
| LFP (LiLO) | Not Detected |
As the table shows, common ternary lithium batteries can enter thermal runaway at temperatures as low as 147°C. The LiLO battery, however, remains stable well beyond these temperatures, making it the safest option against overheating.
A Proven Safety Record
The inherent safety of LiLO chemistry is confirmed by rigorous, independent testing. Industry standards exist to push batteries to their limits and evaluate their fire risk. One of the most important standards in North America is UL 9540A.
What is UL 9540A? UL 9540A is the key safety standard for testing fire propagation in battery energy storage systems. It is not a pass/fail certification but a method to assess risk. The test simulates worst-case scenarios to see if a single failing battery cell will cause a chain reaction (thermal runaway) that spreads to other cells and creates a larger fire.
This testing standard evaluates every component, from the individual cell to the complete battery system, to understand how it behaves under extreme thermal stress. Systems using LiLO chemistry consistently demonstrate superior performance in these tests. They effectively contain thermal events at the cell level and prevent fire from spreading. This proven track record in standardized testing provides clear, third-party validation of the safety claims behind lilo battery technology.
Safety Showdown: LiLO vs. Ternary

Understanding the fundamental science of LiLO safety is one thing. Seeing how it performs against the competition provides a much clearer picture. Ternary lithium batteries, which include common chemistries like NCM (Nickel Cobalt Manganese) and NCA (Nickel Cobalt Aluminum), are widely used. However, their design prioritizes energy density, often at the expense of safety. This section directly compares the risk profiles of these two major lithium battery technologies.
The Risk Profile of Ternary Lithium Batteries
The higher risk associated with ternary lithium batteries comes from their chemical instability. The materials inside these batteries are more likely to react dangerously under stress. Several factors contribute to this heightened risk profile.
- The cathode material in ternary lithium batteries can break down at high temperatures. This process releases pure oxygen. The oxygen then mixes with the flammable liquid electrolyte inside the battery, creating intense heat and fuel for a fire.
- Batteries with a higher nickel content, a common feature in high-performance ternary lithium batteries, begin to overheat at even lower temperatures. This makes them more sensitive to charging issues or hot environments.
- The failure process often starts early. A protective internal layer can begin to break down at temperatures as low as 77°C (171°F), kicking off a heat-generating reaction that can lead to thermal runaway.
This chemical volatility is the core reason why a damaged or overcharged ternary lithium battery poses a significant fire hazard. The battery itself contains both the fuel and the oxygen needed to sustain a fire, making it difficult to extinguish. The design of a lithium polymer battery can influence this, but the core chemistry of ternary lithium remains the primary risk factor.
Direct Safety Metric Comparison
Safety is not just theoretical; it is measured through rigorous “abuse testing.” These tests push a battery to its absolute limits to see how it fails. The results from these physical tests reveal a stark contrast between LiLO and ternary lithium batteries.
One of the most revealing evaluations is the nail penetration test. This test simulates a severe internal short circuit by puncturing the battery with a metal nail.
Overcharging is another common cause of battery failure. A study was conducted to see how different battery types handle being charged beyond their capacity. The research compared the behavior of a ternary lithium battery (NCM), a LiLO battery (LFP), and another lithium chemistry (LCO). The results established that ternary lithium batteries have a lower tolerance for overcharge and a much more destructive failure.
When a LiLO battery is severely overcharged, it shows clear signs of stress.
- The battery swells significantly as internal pressure builds from gas creation.
- These gases include hydrogen, carbon dioxide, and other hydrocarbons.
- This reaction is a failure, but it is a much more controlled and less violent one compared to the alternative.
The lilo battery technology is engineered to fail more predictably. The failure of a ternary lithium battery is often sudden and catastrophic. This makes the choice of lithium chemistry a critical decision for any application where safety is the top priority for a lithium-ion battery. The fundamental design of a lithium polymer battery is important, but the internal chemistry of a lithium polymer battery dictates its ultimate safety.
Key Advantages of LiLO Battery Technology

Safety is a primary benefit, but lilo battery technology offers other major advantages. These strengths make it a practical and responsible choice for many uses. The technology excels in longevity and cost-effectiveness compared to ternary lithium batteries. This makes the battery a smart long-term investment.
Superior Lifespan and Durability
A battery’s lifespan is measured in charge cycles. LiLO batteries offer a significantly longer cycle life than ternary lithium batteries. This durability comes from its robust chemistry. A key factor is its tolerance for a deep Profondeur de déversement (DoD).
Depth of Discharge (DoD) refers to the percentage of a battery’s energy that has been used. A higher DoD puts more stress on a battery, reducing its total lifespan. A LiLO battery handles this stress much better than ternary lithium batteries.
This resilience means you can use more of the battery’s capacity without quickly degrading it. The stable lithium chemistry allows for thousands of cycles. For example, a LiLO battery can achieve many more cycles at a high DoD than typical ternary lithium batteries.
| DoD (%) | Approximate Cycle Life (LiLO) |
|---|---|
| 80% | 3,000 cycles |
| 70% | 4,000 cycles |
| 50% | 5,000 cycles |
This superior cycle life makes this lithium battery a more durable and reliable power source over time than ternary lithium batteries. The longevity of this battery is a key advantage over ternary lithium batteries.
Lower Cost and Ethical Sourcing
LiLO batteries are more affordable than ternary lithium batteries. This cost difference starts at the raw material level. The battery uses iron and phosphate, which are abundant and inexpensive. Ternary lithium batteries rely on costly metals like cobalt and nickel. This price gap is clear when looking at market costs.
| Type de batterie | Average Price (per kWh) |
|---|---|
| LFP (LiLO) | $98.5 |
| NCM | $112.7 |
| ANC | $120.3 |
Beyond price, the sourcing of materials raises ethical questions for ternary lithium batteries. Cobalt mining, essential for many ternary lithium batteries, is linked to serious problems.
- Cobalt mining has faced scrutiny due to child labor.
- Cobalt mining has faced scrutiny due to environmental damage.
Because LiLO chemistry is cobalt-free, it avoids these ethical issues. This makes the lithium battery a more responsible choice than many ternary lithium batteries. Choosing this lithium battery supports a cleaner supply chain compared to ternary lithium batteries.
Understanding the Performance Trade-Offs
While LiLO technology offers superior safety and longevity, it is important to understand its performance trade-offs. No single battery is perfect for every situation. The choice between a LiLO battery and ternary lithium batteries often depends on the user’s priorities. This section examines key differences in energy density, cold weather battery performance, and charging.
Energy Density and Range
The most significant trade-off is energy density. Energy density measures how much power a battery can store for its weight. Ternary lithium batteries have a higher energy density than LiLO batteries. This means ternary lithium batteries can store more energy in the same amount of space. The lower energy density of a LiLO battery directly impacts the range of an electric vehicle.
A vehicle with a LiLO battery pack will generally have a shorter driving range than the same vehicle with a similarly sized pack made from ternary lithium batteries. The difference can be around 33% less range.
The chart below shows the typical energy density for different lithium battery chemistries. The lower energy density is a key factor in battery performance.
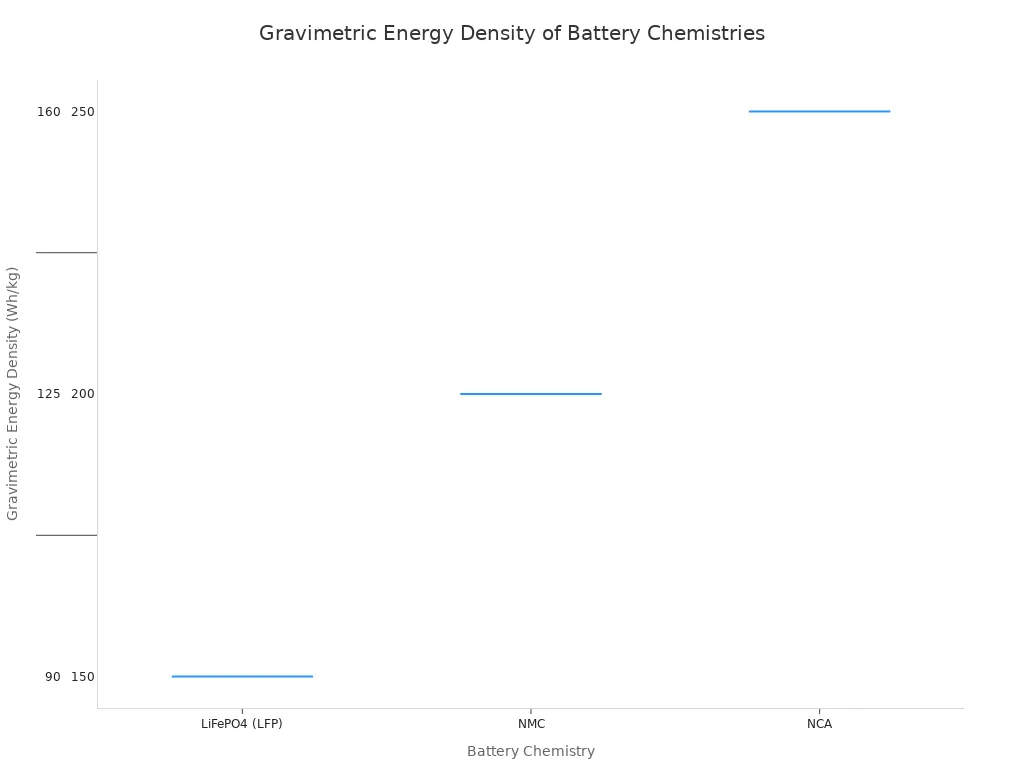
This difference in energy density is a primary reason why some high-performance electric vehicles use ternary lithium batteries. They prioritize maximum range over the benefits of LiLO. The higher energy density of ternary lithium batteries comes with the safety risks discussed earlier.
Cold Weather Performance
Another consideration is cold weather battery performance. The chemistry of a LiLO battery makes it more sensitive to low temperatures. In cold conditions, the battery performance can decrease, and charging can become slower. This is a known challenge for this type of lithium battery.
However, manufacturers have developed smart solutions to this problem.
- Active Heating Systems: Many electric vehicles use heaters or heat pumps to keep the battery at an optimal temperature.
- Heat Recovery: Some systems capture waste heat from the motor to warm the battery, improving efficiency.
- Advanced Management: A Battery Management System (BMS) can adjust charging rates to protect the lithium battery in the cold.
These technologies help improve the battery performance of LiLO systems in colder climates, making them more practical for year-round use. The battery performance of ternary lithium batteries is also affected by cold, but generally to a lesser extent.
Charging Speed and Battery Health
LiLO batteries can typically be charged to 100% without causing significant long-term damage. This is a major advantage over many ternary lithium batteries. Owners of vehicles with ternary lithium batteries are often advised to only charge to 80% for daily use to preserve battery health. The robust nature of LiLO chemistry allows for more flexible and complete charging. This simplifies ownership and ensures the full capacity is available when needed. The higher energy density of ternary lithium batteries does not change this recommendation.
Making the Right Choice
Selecting the right battery technology depends entirely on the user’s priorities. The unique characteristics of LiLO and ternary lithium batteries make them suitable for different applications. Understanding these differences helps consumers make an informed decision.
Ideal Uses for LiLO
The safety, longevity, and cost-effectiveness of LiLO technology make it the ideal choice for many applications where reliability is more important than maximum range.
Real-World Reliability 🏔️ A LiLO-based energy storage system installed at Paiyun Lodge on Taiwan’s Mt. Jade has operated flawlessly since 2016, proving its durability in demanding environments.
Its strengths are especially clear in these areas:
- Stationary Energy Storage: Home and business battery systems benefit from LiLO’s long lifespan and safety. They provide dependable power without the fire risk associated with some ternary lithium batteries.
- Recreational Vehicles (RVs) and Marine Applications: The stable chemistry of this lithium battery is perfect for the confined spaces of RVs and boats. It is lightweight, requires no maintenance, and does not produce hazardous gases.
- Commercial Electric Vehicles: Fleets of delivery vans and city buses use LiLO technology. Companies like Motiv Power Systems equip their medium-duty electric vehicles with this lithium battery. School districts and public transit authorities also choose it for their electric vehicles because safety and long-term operational costs are their main concerns for these applications.
When Ternary Makes Sense
Despite their risks, ternary lithium batteries remain the preferred option for specific high-performance applications. Their main advantage is a higher energy density. This means they can store more power in a smaller, lighter package.
This characteristic is crucial for long-range luxury electric vehicles. For these types of electric vehicles, maximizing the distance on a single charge is the primary design goal. The compact size of ternary lithium batteries allows manufacturers to build powerful electric vehicles without sacrificing interior space or adding excessive weight. While LiLO technology is catching up, ternary lithium batteries currently offer the longest range for passenger electric vehicles. The choice often comes down to a trade-off. Consumers who prioritize maximum range for their electric vehicles may find that ternary lithium batteries better suit their needs for those specific applications, accepting the different safety profile that comes with that lithium battery technology.
The core strength of lilo battery technology is its unmatched safety. This lithium battery offers a long life and lower cost. However, its lower energy density is a trade-off against the higher energy density of ternary lithium batteries. The market for electric vehicles is shifting, with this lithium battery set to surpass 60% of installed capacity. Consumers choosing a battery for electric vehicles must weigh the high energy density of ternary lithium batteries against the safety of this battery. The energy density of ternary lithium batteries suits some electric vehicles, but the energy density of this battery is better for others. Drivers should choose a battery for their electric vehicles based on their needs, considering the energy density of ternary lithium batteries versus the benefits of this lithium battery. The energy density of ternary lithium batteries is a key factor, but not the only one for electric vehicles.
FAQ
Why is a LiLO battery safer than ternary lithium batteries?
A LiLO battery uses stable chemistry. This lithium battery resists overheating. Ternary lithium batteries have a higher fire risk because their chemistry can release oxygen when stressed. The stable LiLO battery design prevents this dangerous reaction, unlike many ternary lithium batteries.
What is the main trade-off with a LiLO battery?
The primary trade-off is lower energy density. A LiLO battery has a lower energy density than ternary lithium batteries. This means the battery stores less power for its size. The higher energy density of ternary lithium batteries gives them a longer range in some applications.
Which battery is better for high performance?
Ternary lithium batteries are often chosen for high performance. Their higher energy density provides maximum range. The energy density of a LiLO battery is lower. The energy density of ternary lithium batteries makes them suitable for luxury electric vehicles where energy density is the top priority.
Is the energy density of a LiLO battery improving?
Yes, the energy density of this lithium battery is improving. New designs are closing the gap with the energy density of ternary lithium batteries. While ternary lithium batteries still lead in energy density, LiLO technology is becoming more competitive for a wider range of applications.

