The lower voltage in a 3.0v lithium battery is not a flaw. This characteristic is a direct result of the specific materials used inside the battery. A battery’s voltage simply measures the difference in electrical potential energy between its negative anode and positive cathode. The unique lithium battery chemistry inside the cells determines this nominal voltage.
A 3.0v lithium battery contains a cathode material that naturally produces a lower voltage. In contrast, a common lithium-ion battery uses different materials in its cells, creating a higher voltage.
Understanding LiFePO4 Lithium Battery Chemistry

Many rechargeable devices use a specific lithium battery chemistry known as Lithium Iron Phosphate, or LiFePO4. These batteries are famous for their exceptional safety and long cycle life. Their characteristic 3.2V nominal voltage is a direct result of the materials used inside their cells.
The Role of the Cathode Material
A battery’s voltage originates from the difference in potential energy between its anode (negative side) and cathode (positive side). The cathode material is the primary factor that sets this voltage. Different chemical compounds possess different electrochemical potentials. A material with a lower potential will naturally produce a lower voltage when paired with the same anode. This is the case with the LiFePO4 lithium-ion battery.
Iron Phosphate’s Lower Potential
The LiFePO4 lithium-ion battery uses a cathode made from Lithium Iron Phosphate. When this cathode is paired with a standard graphite anode, the combination delivers a stable voltage of approximately 3.2V. This is lower than other common lithium-ion battery types because iron phosphate has a lower electrochemical potential compared to materials like cobalt oxide.
This chemistry’s design offers a major advantage: safety. The iron phosphate material is built on an incredibly stable olivine crystal structure. Scientific calculations confirm that LiFePO4 is thermally stable even when fully charged. This strong structure resists breaking down under stress or high temperatures, which greatly reduces the risk of fire and enhances overall safety. This inherent stability makes these cells a top choice for applications where safety is critical.
💡 Did You Know? The strong bonds in the olivine crystal structure make LiFePO4 batteries much less prone to thermal runaway than other lithium chemistries, contributing to their superior safety record.
The Stable Voltage Discharge Curve
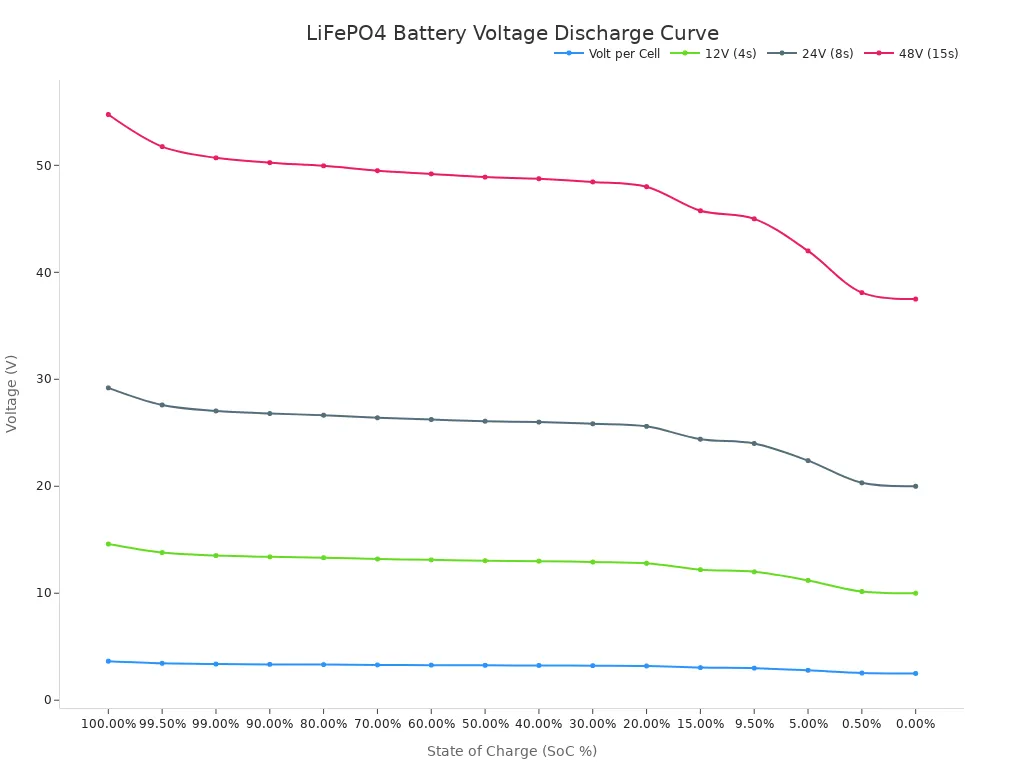
One of the most significant benefits of LiFePO4 chemistry is its remarkably flat discharge curve. This means the battery provides a very stable voltage output throughout most of its discharge cycle. After an initial drop, the voltage of the cells settles between 3.2V and 3.3V and stays there until the battery is nearly empty. This consistent power delivery ensures reliable and long-lasting performance for the connected device.
This flat curve is a key performance characteristic. For example, a LiFePO4 battery maintains a voltage between 3.20V and 3.25V even when it is down to 40% of its charge. This contrasts sharply with other battery types where the voltage drops steadily as they are used. The table below shows how the voltage of LiFePO4 cells corresponds to the State of Charge (SoC) in various battery packs configurations. Notice how the voltage per cell changes very little between 90% and 20% charge. These individual cells are often combined into larger packs to create 12V, 24V, or 48V battery packs.
| SoC% | Volt per Cell | 12V (4s) Packs | 24V (8s) Packs | 48V (15s) Packs |
|---|---|---|---|---|
| 100.00% | 3.65 | 14.6 | 29.2 | 54.75 |
| 90.00% | 3.35 | 13.4 | 26.8 | 50.25 |
| 70.00% | 3.30 | 13.2 | 26.4 | 49.5 |
| 50.00% | 3.26 | 13.04 | 26.08 | 48.9 |
| 30.00% | 3.23 | 12.92 | 25.84 | 48.45 |
| 20.00% | 3.20 | 12.8 | 25.6 | 48.0 |
| 9.50% | 3.00 | 12.0 | 24.0 | 45.0 |
| 0.00% | 2.50 | 10.0 | 20.0 | 37.5 |
The following chart visually demonstrates this flat discharge curve for different LiFePO4 packs. This steady voltage makes this lithium battery chemistry ideal for high-power applications that demand consistent energy, from electric vehicles to large-scale energy storage.
The Primary 3.0v Lithium Battery Explained
Beyond rechargeable packs, another common type of lithium battery is the primary (non-rechargeable) cell. These small, coin-shaped power sources are found in countless everyday devices. The specific lithium battery chemistry inside these cells is designed for long-term, single-use applications, and it inherently produces a lower voltage. A typical 3.0v lithium battery provides reliable power for years.
The Li-MnO2 Chemistry
The most common chemistry for a 3.0v lithium battery is Lithium-Manganese Dioxide (Li-MnO2). These cells use a lithium metal anode and a manganese dioxide cathode. This combination is engineered for stability and a very long shelf life. The design of these cells prioritizes a slow self-discharge rate. This allows a 3.0v lithium battery to sit in a device or package for up to 10 years and still provide power. Their excellent shelf life makes them ideal for low-drain electronics.
You can find these small but powerful cells in a wide variety of devices, including:
- Car key fobs
- Computer motherboards (for CMOS)
- Watches and calculators
- Medical devices like glucometers
- Garage door openers
- Electronic organizers and toys
The 3.0v lithium battery is perfect for these applications because it delivers a consistent voltage over a long period. Unlike rechargeable packs, these cells are meant to be replaced, not recharged.
Anode and Cathode Reaction
The nominal 3.0V voltage of these batteries is a direct result of their internal chemistry. A battery’s voltage is the difference in electrical potential between its anode and cathode. In Li-MnO2 cells, the materials are specifically chosen to create this exact voltage.
- Anode (Negative): The anode is made of pure lithium metal. During discharge, it gives up an electron, becoming a lithium ion. The reaction is:
Li → Li⁺ + e⁻. Lithium metal has a very low standard electrode potential of -3.04V. - Cathode (Positive): The cathode is made of heat-treated manganese dioxide. It accepts the lithium ions and electrons from the anode. The reaction is:
MnO₂ + Li⁺ + e⁻ → LiMnO₂. Manganese dioxide has a standard electrode potential of about +1.23V in certain conditions, but the overall cell reaction in its organic electrolyte results in the ~3.0V output.
The difference between the anode’s and cathode’s potential creates the battery’s voltage. The specific materials in a 3.0v lithium battery naturally produce this electrical potential difference.
Irreversible by Design
A primary 3.0v lithium battery cannot be recharged. The chemical reaction that produces power is irreversible. During discharge, lithium ions embed themselves into the manganese dioxide’s crystal structure. This process permanently alters the cathode’s physical structure.
Scientific analysis shows that this change causes tiny cracks and irreversible phase transitions within the material. Attempting to force the reaction in reverse by applying an external current would not restore the original structure. Instead, it could cause the battery to overheat and fail dangerously. This is why the 3.0v lithium battery is designed for a single, complete discharge cycle. Its long shelf life ensures it is ready when needed. This contrasts with rechargeable battery packs, which are built from cells designed for hundreds or thousands of cycles. Many small devices use single cells, while larger equipment may use packs. These packs are different from rechargeable battery packs. Some specialized equipment uses packs of primary cells for long-term deployment. These packs offer a high energy density and a stable voltage.
Higher Voltage Chemistries: A Comparison

While LiFePO4 and primary lithium cells offer unique benefits at a lower voltage, many consumer electronics rely on a lithium-ion battery with a higher nominal voltage, typically 3.6V or 3.7V. This increased voltage is not arbitrary; it is a direct outcome of using different, more potent cathode materials.
High-Potential Cathode Materials
The key to a higher voltage lies in creating a larger difference in electrical potential between the anode and the cathode. Think of it like two points on a hill. The anode is at the bottom, and the cathode is at the top. The voltage is the distance between them. Cathode materials like Lithium Cobalt Oxide have a higher position on this “hill” than iron phosphate, creating a larger potential gap and, therefore, a higher voltage.
Common high-potential cathode materials produce a higher operating voltage when paired with a graphite anode. This characteristic is fundamental to their design.
| Material del cátodo | Typical Operating Voltage |
|---|---|
| LiCoO₂ (LCO) | ~3.7 V |
| LiMn₂O₄ (LMO) | ~3.8 V |
Cobalt and Nickel Oxides
The most common chemistries for a high-voltage lithium-ion battery are Lithium Cobalt Oxide (LCO) and Nickel Manganese Cobalt (NMC). LCO cells, often found in smartphones and laptops, have a nominal voltage of 3.7V. NMC cells, popular in electric vehicles and power tools, have a nominal voltage of 3.6V. These materials have a layered crystal structure, which differs from the stable olivine structure of LiFePO4. While this structure allows for a high energy density, it is less thermally stable. According to Oregon State University chemistry researcher Xiulei “David” Ji, cobalt is also expensive and toxic, which makes iron-based cells a safer and more sustainable choice for many applications.
Voltage vs. Energy Density
A higher voltage directly contributes to a higher energy density. Energy density (Wh/kg) is the amount of energy a battery can store for its weight. Since energy is calculated by multiplying voltage and capacity, a higher voltage results in more energy for the same capacity. This is why LCO and NMC cells are preferred for small, portable devices where maximizing power in a limited space is critical. For instance, NMC cells can offer a high energy density between 150-220 Wh/kg, whereas LiFePO4 cells typically range from 90-160 Wh/kg. This trade-off between voltage, safety, and cost is why different types of lithium-ion battery cells are chosen for different jobs, from single-cell devices to large battery packs.
A battery’s nominal voltage is a direct result of its internal lithium battery chemistry. The lower voltage in LiFePO4 and a 3.0v lithium battery comes from their unique cathode materials. Voltage is a chemical characteristic, not an indicator of quality. Engineers choose lower-voltage cells for specific benefits like superior safety and a long shelf life. The exceptional safety of a lithium-ion battery like LiFePO4 makes it ideal for applications where safety is paramount.
| Tipo de batería | Ciclo de vida |
|---|---|
| LCO | 400-1200 cycles |
| LiFePO4 (LFP) | ~2000 ciclos |
This focus on safety and longevity is why these cells are used in:
- Vehículos eléctricos
- Medical devices
- Home energy storage systems
The excellent shelf life of a 3.0v lithium battery ensures reliability and safety. Ultimately, the voltage of a lithium-ion battery is a design choice that prioritizes safety and a long shelf life.
PREGUNTAS FRECUENTES
Why is my lithium battery only 3.2V?
A 3.2V battery uses Lithium Iron Phosphate (LiFePO4) chemistry. Its iron phosphate cathode naturally produces a lower voltage compared to other types. This is a deliberate design for superior safety and a long cycle life. Extensive battery testing confirms this stable voltage characteristic.
Can I charge a 3.0V lithium coin cell?
No, you cannot charge a 3.0V primary lithium battery. Its chemistry is irreversible by design. Attempting to recharge it can cause dangerous overheating or rupture. Manufacturers perform strict battery testing to ensure these single-use cells are safe and reliable for their intended purpose.
Is a higher voltage battery always better?
A higher voltage is not always better. Voltage is a chemical trait, not a sign of quality. Lower-voltage LiFePO4 batteries are chosen for their excellent safety and longevity. Engineers use battery testing to match the right voltage and chemistry to an application’s specific needs.
How is battery voltage determined?
A battery’s voltage is the difference in electrical potential between its negative anode and positive cathode. The specific materials used determine this value.
Nota: Professionals use specialized equipment for precise battery testing to verify the voltage and overall performance of a battery cell or pack.

