Mastering lithium-ion battery assembly rests on three pillars: deep component knowledge, a precise understanding of the battery assembly process, and a firm commitment to safety. This expertise is crucial in a dynamic market where battery costs are shifting.
In 2024, battery prices in China fell by nearly 30%. This made a Chinese lithium battery significantly more affordable than alternatives.
The global demand for high-quality lithium-ion batteries fuels immense industry growth. A successful battery assembly transforms raw lithium materials into a finished lithium ion battery. This process is vital as the market for the lithium ion battery continues to expand.
| Metric | Value |
|---|---|
| Market Value (2024) | USD 107.14 billion |
| Projected Market Value (2032) | USD 578.20 billion |
| CAGR (2024-2032) | 23.22% |
Anatomy of a Lithium-Ion Battery

A deep understanding of a lithium-ion battery begins with its internal components. Each part plays a specific role in storing and releasing energy. These components are divided into two main categories: core active materials that drive the electrochemical reactions and structural parts that ensure safety and integrity. A successful lithium battery depends on the precise interplay of these elements.
Core Active Materials
Active materials are the heart of the battery. They are where the chemical reactions happen. The choice of these materials directly defines the battery’s performance, including its energy density, lifespan, and cost.
Anode Materials
The anode is the negative electrode of the battery. It stores lithium ions during charging. Graphite is the most common anode material. Its stable structure and high electrical conductivity make it ideal for this purpose.
- Graphite: The industry standard due to its low cost and reliability.
- Silicon: Offers much higher energy density but can swell during charging, which reduces the battery’s lifespan.
- Graphite-Silicon Blends: Manufacturers often add small amounts of silicon to a graphite anode. This blend boosts energy capacity while managing the swelling issue.
Cathode Chemistries
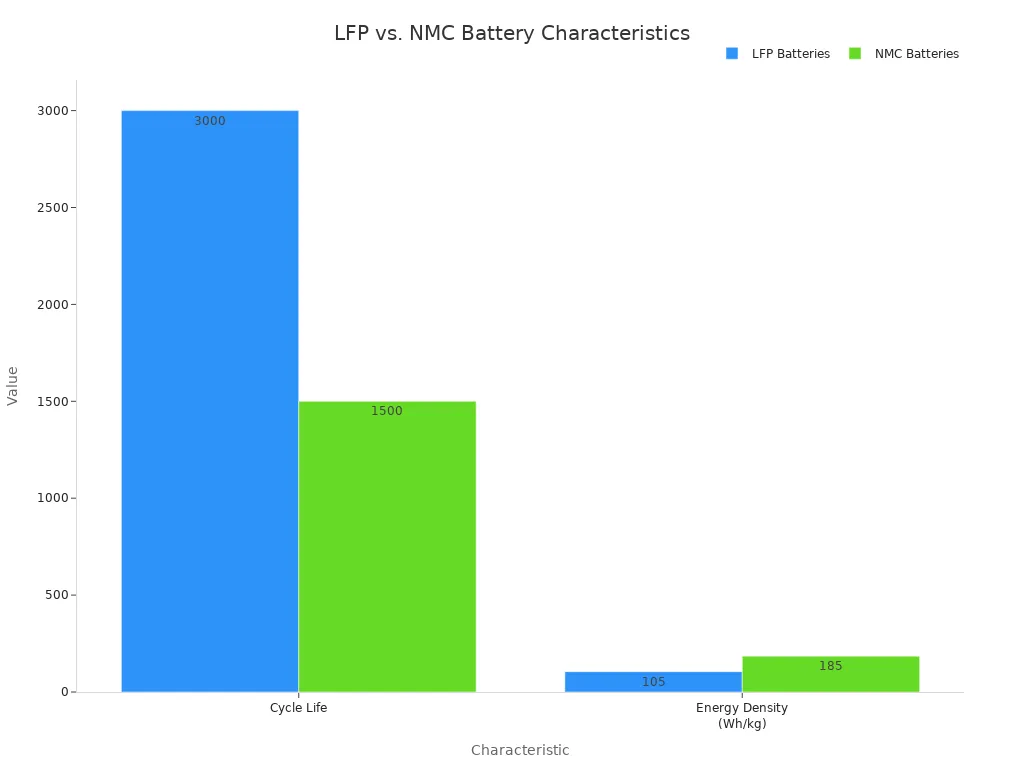
The cathode is the positive electrode. It determines the battery’s capacity and voltage. The specific chemistry of the cathode is a key factor in a lithium ion battery’s performance. Common cathode materials include Lithium Nickel Manganese Cobalt Oxide (NMC) and Lithium Iron Phosphate (LFP). The movement of the lithium ion is central to its function.
Different chemistries offer distinct advantages. For example, LFP is known for its exceptional safety and long cycle life, while NMC provides higher energy density, making it suitable for electric vehicles where range is a priority.
| Charakteristisch | LFP (Lithium Iron Phosphate) | NMC (Lithium Nickel Manganese Cobalt) |
|---|---|---|
| Die Energiedichte | Lower (90-120 Wh/kg) | Higher (150-220 Wh/kg) |
| Zyklus Leben | Excellent (3,000+ cycles) | Good (1,000-2,000 cycles) |
| Sicherheit | Sehr hoch | Good, but lower than LFP |
| Kosten | Unter | Higher (due to cobalt and nickel) |
The Electrolyte Solution
The electrolyte is a chemical medium that allows the lithium ion to flow between the anode and cathode. It is typically a liquid organic solvent containing lithium salts. The electrolyte does not conduct electricity itself, but it is essential for the movement of each lithium ion. This ion transport enables the battery to be charged and discharged.
Structural and Safety Parts
These components do not participate in the chemical reaction. Instead, they provide physical structure and prevent safety hazards like short circuits.
The Separator Film
The separator is a micro-porous membrane. It sits between the anode and cathode. Its main job is to prevent the electrodes from touching, which would cause a short circuit. The separator allows the lithium ion to pass through freely. This makes it a critical safety feature in every lithium battery.
Cell Casing Formats
The cell casing encloses all the internal components, protecting them from moisture and physical damage. There are three primary formats for a lithium ion battery, each with unique trade-offs.
Anmerkung: The choice of casing format impacts the battery pack’s final weight, shape, and thermal management capabilities.
| Cell Type | Vorteile | Benachteiligungen |
|---|---|---|
| Zylindrisch | High mechanical stability, cost-effective, good thermal management. | Lower packing efficiency, heavier for the same capacity. |
| Prismatisch | Space-efficient, high packing density, easier to assemble into packs. | Poorer thermal management, can swell over time. |
| Pouch | Lightweight, flexible design, excellent thermal management. | Low mechanical stability, vulnerable to damage, can swell. |
The Core Battery Assembly Process

The journey from raw materials to a functional power source is a meticulous sequence of engineering steps. The core battery assembly process transforms carefully prepared components into a finished lithium ion battery. This multi-stage procedure demands precision at every turn to ensure the final product is efficient, reliable, and safe. A successful battery assembly hinges on mastering each phase, from creating the electrodes to the final cell finishing.
Electrode Manufacturing
Electrode manufacturing is the foundational stage of the battery assembly process. It involves creating the anode and cathode sheets that will store and release energy. The quality of these electrodes directly impacts the battery’s overall performance and lifespan.
Slurry Mixing
The first step is creating a homogenous mixture called a slurry. This process combines active materials (like graphite or NMC), conductive agents, and binders with a solvent. Precise control over this step is critical for optimal electrode performance. Key parameters include:
- Solids Loading: This is the proportion of solid materials to the liquid solvent. Higher solids loading can increase a battery’s energy density but also makes the slurry thicker and harder to mix.
- Solid-to-Liquid Ratio: A typical ratio is between 50:50 and 60:40. Adjusting this ratio impacts viscosity and how the slurry behaves during coating.
- Sequence of Addition: The order in which technicians add materials to the mixer significantly affects the final quality. A proper sequence ensures all components disperse evenly.
- Temperature Control: Technicians must monitor and control the temperature to prevent the degradation of binders and active materials.
Inline monitoring systems use sensors to track these parameters in real-time, allowing for immediate adjustments to maintain slurry quality.
Electrode Coating
Next, specialized machines apply the slurry onto a metallic current collector foil. The anode slurry goes onto a copper foil, while the cathode slurry is coated onto an aluminum foil. The goal is to apply a perfectly uniform and consistent layer. However, defects can arise during this delicate process.
Watch Out for Defects! Even minor flaws can compromise battery safety and performance. Common issues include:
- Pinholes: Tiny holes caused by bursting bubbles in the slurry.
- Line Defects: Elongated areas with no coating, often caused by impurities blocking the coating die.
- Particle Contamination: Foreign particles from dust or machine wear can get trapped in the coating. Metallic particles are especially dangerous as they can cause short circuits.
Drying and Calendering
After coating, the electrode sheets move through long ovens to evaporate the solvent, leaving a dry, porous layer of active material. Following the drying phase, the electrodes undergo calendering. This step involves passing the sheets through two large, heavy rollers that compress the material. This compression increases the material’s density and improves its electrical contact with the current collector. Calendering is a high-pressure process that can sometimes introduce its own defects, such as particle rupture or fractures in the electrode coating.
Electrode Slitting
The final step in electrode manufacturing is slitting. Here, machines cut the large, coated rolls into smaller, precisely sized strips, or “bobbins,” ready for cell assembly. The quality of the cut is paramount. A poor cut can create microscopic metallic burrs on the edge of the electrode.
These burrs are a major safety concern. If a burr is large enough to puncture the separator film, it can create an internal short circuit, leading to battery failure.
To prevent this, manufacturers follow strict best practices. Advanced slitting methods like laser slitting und ultrasonic slitting offer high precision and minimize dust generation. For traditional blade slitting, key process controls include:
- Blade Condition: Using sharp tungsten carbide blades and rotating them frequently (e.g., every 8 hours) is critical.
- Cut Pressure: Applying the correct lateral force optimizes the cut and reduces burr formation.
- Inspection: Inline camera systems inspect the electrode edges to ensure burr height does not exceed 5μm.
Cell Construction
With the electrodes prepared, the next stage is cell construction. This phase of the battery assembly process involves assembling the anode, cathode, and separator into a “jelly roll” or stack, which forms the core of the battery cell. This is a critical part of the lithium-ion battery assembly.
Stacking and Winding
There are two primary methods for arranging the electrodes for cell assembly: winding and stacking.
- Winding is common for cylindrical and some prismatic cells. A machine winds long strips of the anode, separator, and cathode into a compact “jelly roll.” This method is fast and cost-effective.
- Z-Stacking is used for pouch and many modern prismatic cells. Machines cut the electrodes into individual sheets and stack them in an alternating pattern: anode, separator, cathode, separator, and so on.
While winding is cheaper, Z-stacking offers superior long-term performance. The stable internal structure of a stacked battery reduces the risk of distortion and bulging over thousands of cycles.
Tab Welding
Each electrode needs a conductive tab to connect it to the battery’s external terminals. Tab welding is the process of attaching these metallic tabs to the electrode foils. The integrity of these welds is crucial for the battery’s electrical performance. A poor weld creates high resistance, which generates heat and reduces efficiency. There are several common welding techniques.
| Welding Technique | Die wichtigsten Vorteile | Gemeinsame Anwendungen |
|---|---|---|
| Laser Welding | High speed, high precision, and low heat impact. | EV battery modules, high-performance electronics. |
| Ultrasonic Welding | Energy-efficient, rapid process with no high-temperature melting. | Small lithium ion cells for portable devices. |
| Resistance Welding | Simple, cost-effective, and reliable for mass production. | Large-scale industrial energy storage. |
Cell Encapsulation and Casing
The assembled electrode stack or jelly roll is now ready to be placed into its casing. For pouch cells, the stack is inserted into a flexible, aluminum-laminated pouch. For prismatic and cylindrical cells, it is inserted into a rigid metal can. This step protects the internal components from moisture, oxygen, and physical damage. For pouch cells, the process includes an initial heat seal on three sides, leaving one side open for the next step.
Cell Finishing
The final stage of the battery assembly process is cell finishing. These steps activate the battery’s chemistry and prepare it for use. This is where the raw cell assembly transforms into a functional energy storage device.
Electrolyte Filling
The cell is placed inside a vacuum chamber. A machine then injects a precise amount of liquid electrolyte, which is the medium that allows lithium ion transport. The vacuum helps the electrolyte fully soak into the porous electrodes and separator, ensuring no air bubbles are trapped inside. Specialized vacuum filling equipment is essential for this step in high-volume manufacturing of a lithium battery. This process is vital for the movement of each lithium ion.
Formation and Aging
Formation is the battery’s first-ever charge and discharge cycle. This is arguably the most critical step in the entire battery assembly. During this initial charge, a crucial chemical reaction occurs at the surface of the anode.
- The positive electrode releases lithium ions.
- These lithium ions travel through the electrolyte to the negative electrode.
- The lithium ions react with the electrolyte to create a protective layer called the Solid Electrolyte Interphase (SEI) film.
This SEI film is essential. It stabilizes the battery, protects the anode from further reactions, and enables the battery to function as a rechargeable device. After formation, the lithium battery is left to rest, or “age,” for a period. This allows the SEI layer to stabilize.
Degassing and Final Sealing
The formation process generates gases (like CO2 and H2) as a byproduct. These gases must be removed, as they can hinder lithium ion flow and create internal pressure, posing a safety risk. In the degassing step, the cell is punctured in a controlled environment to release the trapped gases. After degassing, the cell is permanently sealed. This final hermetic seal is vital to prevent any electrolyte leakage and protect the internal chemistry, ensuring the longevity and safety of the lithium battery.
Quality in Lithium-Ion Battery Assembly
Achieving excellence in lithium-ion battery assembly requires non-negotiable quality and safety practices. These protocols ensure every lithium battery is reliable and safe. The process combines advanced analytical techniques with specialized equipment for robust quality assurance. Expert strategies in parameter interpretation enhance the performance and life of each battery.
Quality Control and Testing
Rigorous testing at every stage of battery assembly is essential. Manufacturers use a series of checks to identify and correct defects before they compromise the final product.
Controlled Dry Room Environments
The presence of moisture is extremely harmful to lithium chemistry. For this reason, the entire battery assembly process occurs inside controlled dry rooms. These environments maintain exceptionally low humidity levels to prevent unwanted chemical reactions with the lithium components. The specifications are strict, as even tiny amounts of water can degrade a battery.
Critical Environment Specs: The dew point in a dry room is a key metric. A lower dew point means less moisture in the air.
| Stage/Type | Dew Point (dp) | Relative Humidity (RH) |
|---|---|---|
| General Cell Assembly | -35°C to -45°C | < 2% |
| Next-Gen Lithium-ion | -60.0°C | K.A. |
| Electrolyte Filling | Below -80.0°C | K.A. |
In-Process Inspections
Technicians perform continuous inspections throughout production. During electrode manufacturing, they monitor slurry viscosity and coating thickness to ensure uniformity. In cell construction, precise stacking alignment is critical. Misaligned electrodes can extend beyond the separator, creating a risk of short circuits. Advanced methods like X-ray imaging allow inspectors to see inside the sealed battery, checking for internal structural defects before the battery moves to the next stage.
End-of-Line Electrical Tests
After a lithium battery is fully assembled, it undergoes a final set of electrical tests. These checks confirm the battery meets performance and safety standards. Key tests include:
- Open Circuit Voltage (OCV): This simple measurement reveals the battery’s state of charge. An OCV outside the target range indicates a potential problem with the lithium ion chemistry.
- AC Internal Resistance (ACIR): This fast test measures the opposition to current flow inside the battery. A high ACIR value often points to a manufacturing defect or poor weld quality.
Critical Safety Protocols
Safety is the top priority in any lithium-ion battery assembly operation. Strict protocols govern the handling of materials and the prevention of common hazards.
Hazardous Material Handling
Technicians must handle reactive lithium materials and flammable electrolytes with extreme care. Key safety procedures involve preventing overcharging, avoiding overheating, and ensuring proper storage conditions. If an abnormal situation like smoke or leakage occurs, operators must stop using the battery and handle it according to established safety regulations.
Contamination Control
Foreign particles are a major threat to battery safety. Metallic contamination, such as burrs from slitting or chips from machinery, can puncture the separator and cause an internal short circuit. Nonmetallic particles like dust or fibers can also interfere with the flow of the lithium ion. A clean manufacturing environment is essential for producing a safe lithium battery.
Short Circuit Prevention
Engineers design the battery to prevent short circuits. They use high-temperature resistant ceramic separators and flame-retardant electrolytes to suppress the growth of lithium dendrites. Additionally, a Battery Management System (BMS) monitors the battery’s status. The BMS can detect a potential short circuit and take action to eliminate the hazard, protecting the battery and the end-user.
The Future of Battery Assembly
The future of battery assembly is evolving rapidly. Smart manufacturing and next-generation materials are reshaping the entire production landscape. These advancements promise to make the lithium-ion battery more powerful, safer, and easier to produce. The industry is moving toward a more automated and intelligent battery assembly process.
Automation and Smart Manufacturing
Automation is the key to scaling up production while maintaining high quality. Robots and artificial intelligence are becoming standard on the factory floor, transforming the battery assembly process from a series of manual tasks into a highly efficient, data-driven operation.
Robotics for Precision and Throughput
Robots now handle many delicate and repetitive tasks in the lithium-ion battery assembly line. This automation increases both speed and precision. Key automated tasks include:
- Incoming material handling and sorting
- Electrode coating and cell stacking
- Laser welding for connections
- Final electrical testing and laser marking
This level of automation in battery assembly ensures each battery is built to exact specifications, reducing human error and boosting overall throughput.
AI in Quality Inspection
Artificial intelligence (AI) enhances quality control during cell assembly. AI-powered computer vision systems scan electrodes at high speeds, identifying critical defects like coating inconsistencies or contamination. During cell stacking, these systems guide robotic arms to ensure perfect alignment of each layer. This real-time inspection catches problems early, which reduces waste and improves the quality of the final lithium battery.
Assembling Next-Gen Lithium-Ion Batteries
Manufacturers are also preparing for new battery chemistries. Innovations in materials like silicon and the development of solid-state designs require significant changes to the traditional cell assembly process.
Innovations in Silicon Anodes
Silicon anodes promise to dramatically increase a battery’s energy capacity. However, they present a major manufacturing challenge. Silicon’s volume can expand up to 300% during charging.
This repeated expansion and contraction causes the anode material to break down. This breakdown leads to a rapid loss of battery capacity und consumes large amounts of active lithium and electrolyte, which shortens the battery’s life.
Engineers are developing new binder materials and electrode structures to manage this issue for the lithium battery.
Preparing for Solid-State Designs
Solid-state lithium-ion batteries replace the liquid electrolyte with a solid material. This change improves safety and energy density. The battery assembly for these designs requires new equipment. Existing machines for liquid-based batteries are not suitable for handling solid materials. The process will still involve stacking electrodes and electrolytes, but it will demand much stricter process controls to prevent damage to the fragile solid layers and ensure a strong bond between them for efficient ion transfer.
Module and Pack Battery Assembly
The final steps in production involve integrating individual cells into larger modules and packs. This stage of battery assembly focuses on mechanical stability and electrical performance.
Cell-to-Module Integration
Proper thermal management is critical when combining cells into a module. Engineers use Thermal Interface Materials (TIMs) to help transfer heat away from the battery.
TIMs, such as thermal pastes or gap fillers, ensure uniform heat distribution. This prevents overheating and optimizes the charging performance of the battery. Strategic spacing between cells also helps control temperature, ensuring every lithium battery operates safely.
Electrical and Mechanical Connections
Robust connections are essential for a reliable battery pack. Manufacturers use customized conductors like copper or aluminum busbars to link the cells. These components are attached using advanced welding techniques.
| Welding Method | Primary Use Case |
|---|---|
| Laser Welding | Connecting busbars with high precision. |
| Ultrasonic Welding | Attaching conductors directly to cell electrodes. |
These methods create strong, low-resistance connections, ensuring efficient power flow and mechanical durability for the finished lithium ion battery.
Successful lithium-ion battery assembly is a systematic discipline. It synthesizes material science, precision engineering, and rigorous process control. Mastering every step of the battery assembly process is fundamental to producing a high-performance, reliable battery. This prevents dangerous defects, like those seen in major battery recalls. Manufacturers can use this guide to refine their battery assembly. They should also embrace emerging technologies. Automation and new battery designs are shaping the industry’s future, promising a safer and more efficient battery for everyone.
FAQ
What is the Solid Electrolyte Interphase (SEI) layer?
The SEI layer is a protective film. It forms on the anode during the battery’s first charge. This critical layer stabilizes the anode. It also prevents unwanted chemical reactions with the electrolyte. A stable SEI layer ensures a long and safe battery life.
Why is a dry room necessary for battery assembly?
Moisture is extremely harmful to lithium-ion chemistry. It can degrade materials and cause safety issues. Manufacturers assemble batteries in special dry rooms. These rooms have very low humidity. This controlled environment protects the battery components and ensures high quality.
What is the difference between winding and stacking? ⚙️
Winding and stacking are two methods for assembling electrodes.
- Winding rolls long electrode sheets into a “jelly roll” for cylindrical cells.
- Z-Stacking layers individual electrode sheets for pouch and prismatic cells.
Stacking offers better long-term stability, while winding is often faster.
Which cell format is the best: cylindrical, prismatic, or pouch?
Each format has unique trade-offs. The “best” choice depends on the application.
Cylindrical cells are durable and cost-effective. Prismatic cells are space-efficient. Pouch cells are lightweight and flexible. Engineers select the format that best meets the product’s specific needs for weight, space, and cost.
What is the most common cause of an internal short circuit?
Internal short circuits often happen when the separator film is damaged. Tiny metallic particles, called burrs, from the electrode slitting process can puncture the separator. This allows the anode and cathode to touch, creating a dangerous short circuit inside the lithium battery.
Why is degassing an important step? 🔋
The formation process creates unwanted gases inside the cell. These gases increase internal pressure and can interfere with ion flow. Degassing removes these trapped gases. This step is essential for the safety, performance, and long-term reliability of the finished lithium ion battery.

