
Die 3,7V Standardspannung in einer Lithium-Ionen-Batterie entsteht durch die einzigartige Chemie zwischen Lithium-Kobalt-Oxid und Graphit-Elektroden. Diese Spannung ist nicht zufällig. Sie ergibt sich vielmehr aus den elektrochemischen Eigenschaften dieser Materialien. Die Standardspannung von 3,7 V prägt das Batteriedesign und die Systemanforderungen und verleiht Lithium-Ionen-Batteriezellen eine höhere Energiedichte und eine längere Laufzeit pro Ladung. Geräte wie Smartphones, Elektrofahrzeuge und tragbare Elektronikgeräte verlassen sich auf diese wiederaufladbare Akkuchemie für effiziente, leichte Energie. Die 3,7-V-Standardspannung treibt auch den globalen Markt für wiederaufladbare Batterietechnologie an, wie die folgende Tabelle zeigt nachstehende Tabelle:
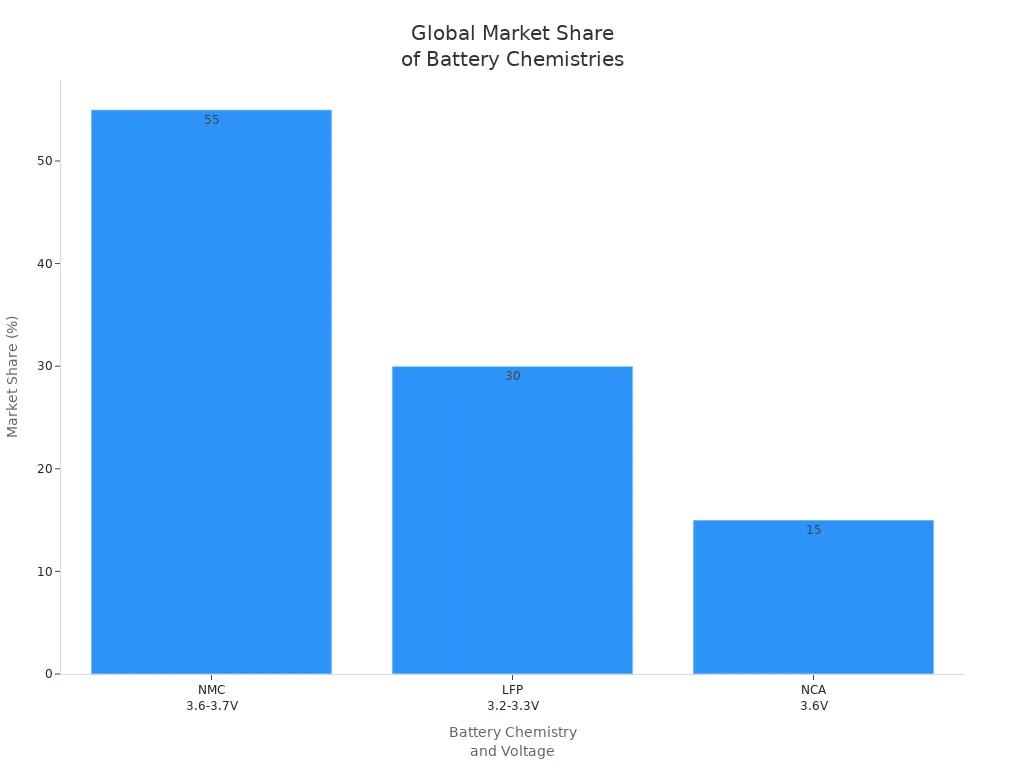
Wenn man versteht, warum diese Spannung so wichtig ist, kann man die Sicherheit von Batterien, die Kompatibilität von Geräten und die Auswahl von Batteriedesigns erklären, die die täglich verwendete Akkutechnologie prägen.
3.7V Standardspannung erklärt

Nominale vs. maximale Spannung
Die Standardspannung von 3,7 V in einer Lithium-Ionen-Batterie wird als Nennspannung bezeichnet. Dieser Wert stellt die durchschnittliche Spannung während des Entladezyklus der Batterie. Ingenieure verwenden die Nennspannung für die Entwicklung von Geräten und Akkus, weil sie die Berechnungen vereinfacht und die Kompatibilität gewährleistet.
- Die Nennspannung ist kein fester Wert. Es handelt sich um einen Durchschnittswert, der Nutzern und Ingenieuren hilft zu verstehen, wie die Batterie während der meisten Zeit ihres Einsatzes funktionieren wird.
- Die tatsächliche Spannung eines Lithium-Ionen-Akkus ändert sich je nach Ladezustand, Stromstärke, Temperatur und Alter des Akkus.
- Die Nennspannung ist wichtig für die Normung. Sie ermöglicht es, dass verschiedene Batterien und Geräte sicher und effizient zusammenarbeiten können.
- Bei Akkupacks wird die Nennspannung verwendet, um zu bestimmen, wie viele Zellen in Reihe geschaltet werden sollen. Drei Zellen mit einer Standardspannung von 3,7 V ergeben zum Beispiel einen Akku mit 11,1 V.
Die In der nachstehenden Tabelle werden die Nenn-, Höchst- und Mindestspannungen verglichen für gängige Batterien auf Lithiumbasis:
| Chemie | Nennspannung (V) | Maximale Spannung (V) | Mindestspannung (V) |
|---|---|---|---|
| LiCoO2 (Lithium-Kobalt-Oxid) | 3.6 - 3.7 | 4.2 | 3.0 |
| LiFePO4 (Lithium-Eisen-Phosphat) | 3.2 | 3.6 | 2.5 |
Anmerkung: Die Nennspannung ist die durchschnittliche Betriebsspannung. Die maximale Spannung ist die höchste sichere Ladespannung und die minimale Spannung ist die niedrigste sichere Entladespannung. Das Einhalten dieser Grenzwerte schützt die Batterie und verlängert ihre Lebensdauer.
Ein Lithium-Ionen-Akku hat bei voller Ladung normalerweise eine maximale Spannung von etwa 4,2 V. Wenn sich der Akku entlädt, sinkt die Spannung. Wenn die Spannung den sicheren Mindestwert erreicht, in der Regel etwa 3,0 V, muss die Batterie wieder aufgeladen werden. Die Standardspannung von 3,7 V liegt zwischen diesen beiden Punkten und spiegelt die typische Leistung des Akkus während des Gebrauchs wider.
Warum 3,7 V gewählt wurde
Die Standardspannung von 3,7 V ist nicht zufällig. Sie ergibt sich aus der Chemie im Inneren der Lithium-Ionen-Batterie, insbesondere aus der Kombination von Lithium-Kobalt-Oxid als Kathode und Graphit als Anode. Diese Chemie erzeugt eine elektrochemische Potenzialdifferenz, die sich bei normalem Gebrauch auf etwa 3,7 V einpendelt.
- Die Standardspannung von 3,7 V entspricht dem durchschnittliche Spannung bei etwa 50% Ladezustand für Lithium-Ionen-Batterien. Dieser Wert bietet ein gutes Gleichgewicht zwischen Energie, Sicherheit und Batterielebensdauer.
- Die Standardspannung von 3,7 V ermöglicht eine hohe Energiedichte, was bedeutet, dass die Batterie mehr Energie auf kleinerem Raum speichern kann. Dies macht Lithium-Ionen-Batterien ideal für tragbare und wiederaufladbare Geräte.
- Viele elektronische Komponenten und Batteriemanagementsysteme sind für die Standardspannung von 3,7 V ausgelegt. Dies vereinfacht die Entwicklung und Herstellung von Geräten.
- Die Standardspannung von 3,7 V unterstützt auch modulare Akkupack-Designs. Mehrere Zellen können in Reihe geschaltet werden, um höhere Spannungen für größere Geräte, wie Elektrofahrzeuge oder Elektrowerkzeuge, zu erzeugen.
- Die weltweite Einführung der Standardspannung von 3,7 V hat zu einer Massenproduktion, niedrigeren Kosten und einer zuverlässigen Lieferkette für Lithium-Ionen-Batterien geführt.
Nicht alle Lithiumbatterien verwenden die Standardspannung von 3,7 V. Zum Beispiel haben Lithium-Eisen-Phosphat-Batterien (LiFePO4) eine Nennspannung von etwa 3,2 V. Die niedrigere Spannung ergibt sich aus ihrer anderen Chemie, die eine höhere Sicherheit und eine längere Lebensdauer bietet, aber eine geringere Energiedichte als herkömmliche Lithium-Ionen-Batterien.
| Batteriechemie | Nennspannung | Volle Ladung Spannung | Entladespannungsbereich |
|---|---|---|---|
| Li-Ion (Standard) | 3.7V | 4.2V | 3,0 V - 4,2 V |
| LiPo | 3.7V | 4.2V | 3,0 V - 4,2 V |
| 18650 | 3.7V | 4.2V | 2,5V - 4,2V |
| LiFePO4 | 3.2V | 3.6V | 2,5V - 3,6V |
Die Standardspannung von 3,7 V verleiht Lithium-Ionen-Akkus eine flaches Spannungsprofil während der Entladung. Dies bedeutet, dass die Batterie während des größten Teils ihres Zyklus gleichbleibende Energie liefert, im Gegensatz zu anderen wiederaufladbaren Batterien wie Nickel-Cadmium oder Blei-Säure, die eine starker Spannungsabfall wenn sie sich entladen. Diese konstante Ausgangsspannung trägt dazu bei, dass die Geräte reibungslos und effizient funktionieren.
Die Aufrechterhaltung einer stabilen 3,7-V-Standardspannung während der gesamten Lebensdauer der Batterie kann eine Herausforderung darstellen. Die die Spannung ändert sich natürlich beim Laden und Entladen der Batterie. Eine Überladung über 4,2 V oder eine Tiefentladung unter 3,0 V kann die Batterie beschädigen, ihre Energiekapazität verringern und ihre Lebensdauer verkürzen. Batteriemanagementsysteme spielen eine wichtige Rolle bei der Überwachung und Steuerung der Spannung, damit die Batterie sicher und zuverlässig bleibt.
Lithium-Ionen-Akku-Chemie

Elektrodenmaterialien
Die Lithium-Ionen-Batterie erhält ihre Standardspannung durch die besonderen Eigenschaften ihrer Elektrodenmaterialien. Die gebräuchlichste Kombination verwendet Lithiumkobaltoxid als Kathode und Graphit als Anode. Diese Materialien erzeugen einen Unterschied im elektrochemischen Potenzial, der die Spannung in der Nähe von 3.7V.
- Die Lithium-Kobaltoxid-Kathode hat ein Elektrodenpotential von etwa 3,9-4,0 V.
- Die Graphitanode hat ein viel niedrigeres Potenzial, etwa 0,1-0,2 V.
- Die Lithiumionen bewegen sich während des Ladens und Entladens zwischen diesen beiden Elektroden, wodurch die Batterie Energie speichern und abgeben kann.
- Die Spannung des Lithium-Ionen-Akkus ergibt sich aus der Differenz zwischen dem Kathoden- und dem Anodenpotenzial.
Dieser Aufbau verleiht der Lithium-Ionen-Batterie ein ausgewogenes Verhältnis von Energiedichte, Stabilität und Sicherheit. Höhere Spannungen liefern zwar mehr Energie, können aber die Sicherheit beeinträchtigen. Niedrigere Spannungen machen die Batterie sicherer, verringern aber ihre Energiedichte. Die Standardspannung von 3,7 V eignet sich für die meisten Geräte, da sie eine gute Mischung aus diesen Eigenschaften bietet.
Die Schichtstruktur von Lithium-Kobalt-Oxid lässt Lithium-Ionen leicht ein- und auswandern. Diese Bewegung wird als reversible Interkalation bezeichnet. Sie trägt dazu bei, dass die Batterie länger hält und die Spannung während des Gebrauchs stabil bleibt.
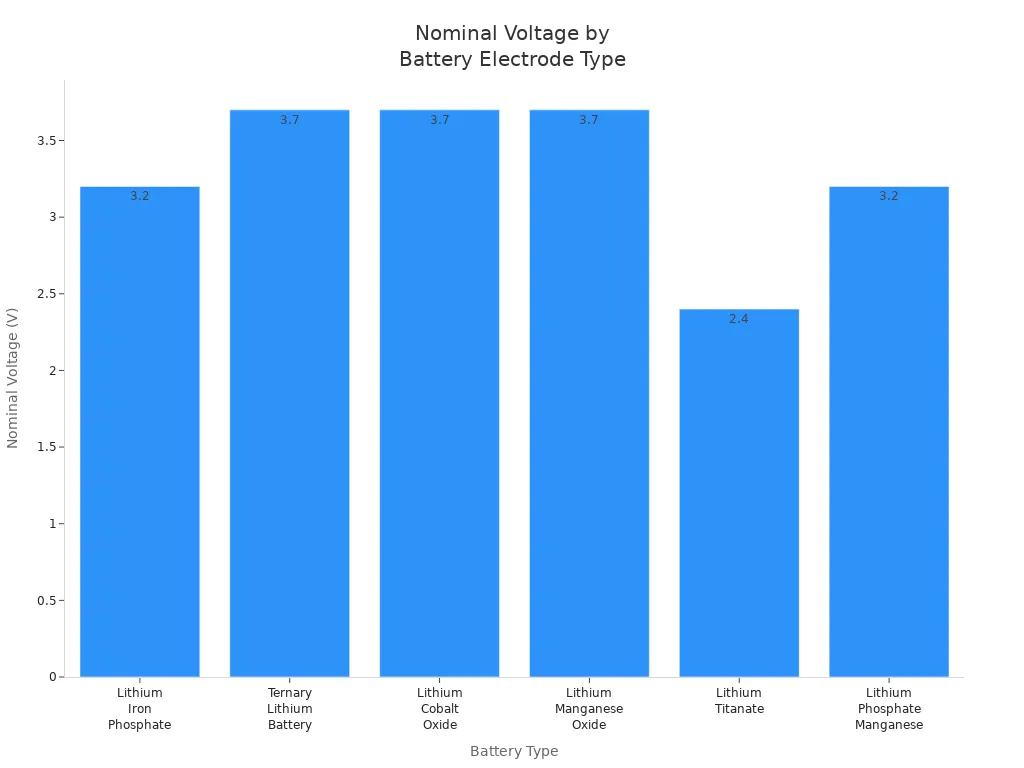
Bei den verschiedenen Batteriearten werden unterschiedliche Materialien verwendet, wodurch sich die Spannung ändert. Lithium-Eisenphosphat-Batterien beispielsweise verwenden LiFePO4 als Kathode und haben eine niedrigere Nennspannung von etwa 3,2 V. Die folgende Tabelle zeigt, wie das Material der positiven Elektrode die Nennspannung beeinflusst:
| Akku-Typ | Material der positiven Elektrode | Nennspannung (V) |
|---|---|---|
| Lithium-Eisen-Phosphat (LiFePO4) | LiFePO4 | 3.2 |
| Ternäre Lithium-Batterie (NCM/NCA) | Lithium-Nickel-Kobalt-Mangan-Oxid | 3.7 |
| Lithium-Kobalt-Oxid (LiCoO2) | LiCoO2 | 3.7 |
| Lithium-Mangan-Oxid (LiMn2O4) | LiMn2O4 | 3.7 |
| Lithium, Nickel, Mangan, Kobalt (NMC) | LiNiMnCoO2 | 3.6 - 3.7 |
| Lithiumtitanat (Li4Ti5O12) | Li4Ti5O12 | 2.4 |
| Lithium-Phosphat-Mangan (LiMnPO4) | LiMnPO4 | 3.2 |
Spannungskurve und Energiedichte
Die Spannungskurve einer Lithium-Ionen-Batterie zeigt, wie sich die Spannung beim Laden und Entladen der Batterie ändert. Diese Kurve hängt davon ab, wie sich die Lithium-Ionen in die Elektrodenmaterialien hinein- und herausbewegen. Wenn die Batterie geladen wird, verlassen die Lithiumionen die Kathode und treten in die Anode ein. Wenn sich die Batterie entlädt, wandern die Ionen zurück zur Kathode. Dieser Vorgang wird als Interkalation bezeichnet.
Die Spannungskurve wird durch die Art und Weise bestimmt, wie sich die Fermi-Niveaus der Elektrodenmaterialien während des Ladens und Entladens ausrichten. Die externe Spannung entspricht der Differenz dieser Energieniveaus. Manchmal zeigt die Kurve kleine Einbrüche oder Anstiege aufgrund von Veränderungen im Inneren der Batterie, wie Phasengrenzen oder Konzentrationsgradienten. Diese Merkmale können zu Effekten wie Spannungshysterese oder Memory-Effekt führen.
Die Energiedichte einer Lithium-Ionen-Batterie gibt an, wie viel Energie sie im Verhältnis zu ihrem Gewicht speichern kann. Die meisten Lithium-Ionen-Batterien haben eine Energiedichte zwischen 120 und 170 Wh/kg. Diese hohe Energiedichte macht sie zu einem beliebten Produkt für tragbare Elektronik, Elektrofahrzeuge und viele andere Anwendungen. Die Batterie kann über den größten Teil ihres Zyklus hinweg gleichmäßig Energie liefern, was zu einem reibungslosen Betrieb von Geräten beiträgt.
Es besteht jedoch ein Kompromiss zwischen Energiedichte und Zyklusdauer. Batterien mit höherer Energiedichte haben oft eine kürzere Lebensdauer. Zum Beispiel:
| Batteriechemie | Nennspannung | Energiedichte (Wh/kg) | Zyklus Lebensdauer (Zyklen) | Wichtigster Kompromiss / Anmerkungen |
|---|---|---|---|---|
| Li-Ion mit hoher Energiedichte | ~3.7V | 120-170 | 500-1000 | Höhere Energiedichte führt zu schnellerer Degradation |
| LiFePO4 (LFP) | ~3.2V | 160-180 | 2000-4000+ | Geringere Energiedichte, aber viel längere Zykluslebensdauer |
- Bei Batterien mit höherer Energiedichte laufen intensivere chemische Reaktionen ab, wodurch sie schneller verschleißen können.
- Lithium-Ionen-Zellen mit hoher Energiedichte halten in der Regel 500 bis 1000 Zyklen.
- Chemikalien mit geringerer Energiedichte wie LFP können mehr als 2000 und manchmal sogar mehr als 4000 Zyklen überstehen.
- LFP-Batterien haben eine geringere Energiedichte, bieten aber mehr Sicherheit und eine längere Lebensdauer.
Innerer Widerstand wirkt sich auch auf die Spannungsstabilität und Zyklenlebensdauer einer Lithium-Ionen-Batterie aus. Wenn die Batterie altert oder hohen Temperaturen ausgesetzt ist, steigt ihr Innenwiderstand. Dies führt zu Spannungseinbrüchen bei starker Belastung, erhöhter Wärmeentwicklung und schnellerem Kapazitätsverlust. Die folgende Tabelle erklärt, wie Innenwiderstand beeinträchtigt die Batterieleistung:
| Aspekt | Wirkung des Innenwiderstands | Beispiel Batteriechemie Auswirkungen |
|---|---|---|
| Spannungsstabilität | Ein erhöhter Innenwiderstand führt zu Spannungsabfällen (Spannungsabfall) bei hoher Strombelastung, wodurch die Ausgangsleistung verringert und das Gerät abgeschaltet werden kann. | LiFePO4 weist einen niedrigen Widerstand auf, der eine stabile Spannung ermöglicht; LCO und NMC zeigen einen schnellen Widerstandsanstieg, der zu Spannungsinstabilität führt. |
| Wärmeerzeugung | Ein höherer Widerstand führt zu mehr Wärme beim Laden/Entladen, was den chemischen Abbau und den Kapazitätsverlust beschleunigt. | NMC-Batterien müssen aufgrund der Widerstandswärme gekühlt werden; LiFePO4 erzeugt weniger Wärme, was die Lebensdauer verlängert. |
| Zyklus Leben | Die Zunahme des Widerstands verkürzt die Lebensdauer der Batterie, indem sie die Degradation beschleunigt. | LiFePO4: 2000-5000 Zyklen mit geringem Widerstand; LCO: 500-1000 Zyklen mit schnellerem Widerstandsanstieg. |
| Einfluss der Temperatur | Hohe Temperaturen beschleunigen das Wachstum des Widerstands, niedrige Temperaturen erhöhen die Impedanz der Ladungsübertragung, was beides die Leistung beeinträchtigt. | Der Widerstand steigt bei hohen Temperaturen schneller an, was die Lebensdauer des Zyklus verringert. |
| Überwachung und Verwaltung | Die Überwachung des Innenwiderstands über die Kapazitätsdämpfungsrate und den Gleichstromwiderstand hilft bei der Vorhersage des Batteriezustands und der Optimierung der Leistung. | Die richtige Konstruktion und Temperaturkontrolle sorgen für einen niedrigen Widerstand, was die Spannungsstabilität und die Lebensdauer der Zyklen erhöht. |
Die Kontrolle der Temperatur, die Vermeidung von Tiefentladungen und die Verwendung intelligenter Ladegeräte helfen, den Innenwiderstand niedrig zu halten. Dadurch wird die Lebensdauer der Batterie verlängert und die Spannung stabil gehalten.
Die Lithium-Ionen-Batterie zeichnet sich durch eine hohe Energiedichte, eine gleichmäßige Spannungskurve und eine gute Zykluslebensdauer aus. Die Wahl der Elektrodenmaterialien, die Art und Weise, wie sich die Lithium-Ionen bewegen, und die Kontrolle des Innenwiderstands machen dies möglich. Dieses Gleichgewicht ist der Grund, warum die Lithium-Ionen-Batterie zum Standard für so viele moderne Geräte geworden ist.
Akkuleistung und Kompatibilität
Integration von Geräten
Die Standardspannung von 3,7 V in einem Lithium-Ionen-Akku ermöglicht eine höhere Energiedichte, was ihn zu einer guten Wahl für moderne Geräte macht. Mit dieser Spannung kann jede Zelle mehr Energie speichern und abgeben als ältere chemische Systeme wie Nickel-Metallhydrid. Die Website Die folgende Tabelle zeigt, wie Lithium-Ionen-Batteriezellen im Vergleich zu NiMH-Batterien in Bezug auf Spannung, Energiedichte und Leistungsdichte abschneiden:
| Merkmal | NiMH-Batterien | Lithium-Ionen-Batterien |
|---|---|---|
| Spannung pro Zelle | 1.25 V | Normalerweise 3,7 V |
| Energiedichte | 55-110 Wh/kg | 100-300 Wh/kg |
| Leistungsdichte | 100-500 W/kg | 500-5000 W/kg |
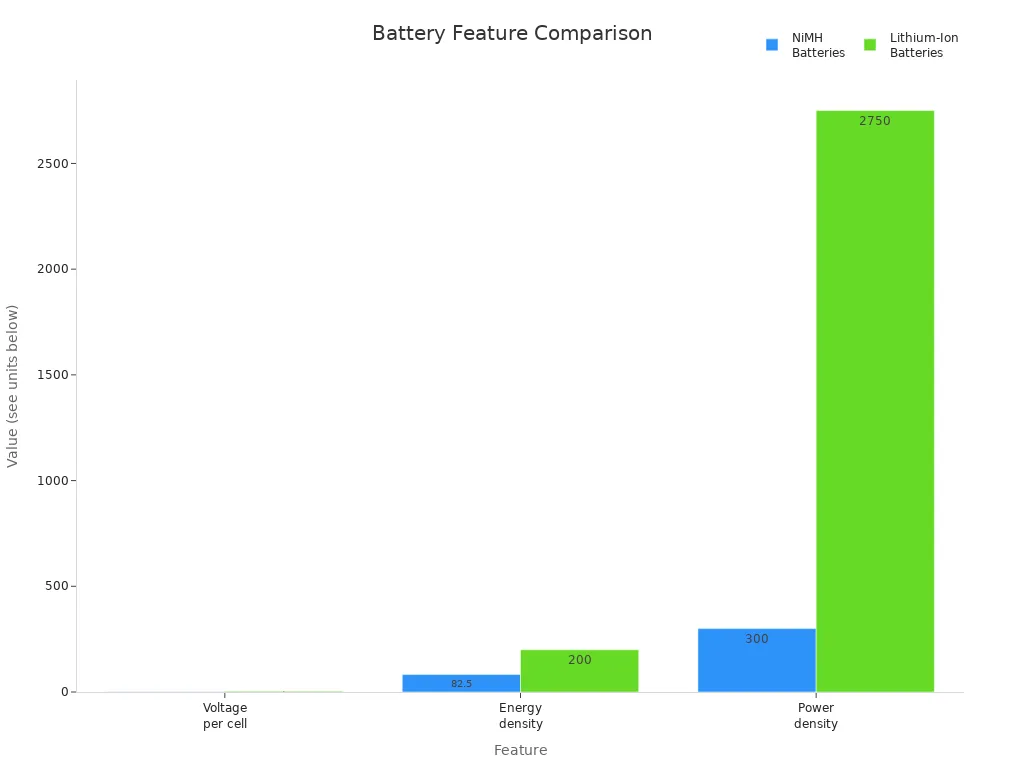
Ein Lithium-Ionen-Akku mit einer Die Spannung von 3,7 V entspricht den Anforderungen vieler wiederaufladbarer Geräte. Gerätehersteller wählen diese Batterie, weil sie dem Spannungsbereich entspricht, den die meisten elektronischen Geräte benötigen. Die höhere Energiedichte bedeutet, dass die Geräte mit einer einzigen Ladung länger laufen können. Batteriemanagementsysteme helfen, die Spannung zu regulieren und Gefahren zu vermeiden, was die Batteriesicherheit und die Batterieleistung verbessert. Das physikalische und chemische Design der Batterie, einschließlich des Elektrolyts und des Separators, gewährleistet eine stabile Energielieferung und Sicherheit. Die Hersteller verwenden außerdem Schutzleiterplatten und kundenspezifische Anschlüsse um die Batterie an das Design des Geräts anzupassen. Sie befolgen strenge Zertifizierungen und Normen, um die Sicherheit und Kompatibilität der Batterien zu gewährleisten.
Sicherheit und Lebenserwartung
Die Sicherheit von Batterien hat bei der Entwicklung von Lithium-Ionen-Batterien oberste Priorität. Eine Überladung über 4,2 V kann zu chemischer Instabilität, Anschwellen oder sogar Explosionen führen. Um dies zu verhindern, enthält jede wiederaufladbare Batterie Überladeschutzschaltungen. Diese Schaltungen überwachen die Spannung und beenden den Ladevorgang, wenn die Batterie 4,2 V erreicht. Einige Systeme verfügen über Temperatursensoren und Strombegrenzer für zusätzliche Sicherheit. Intelligente Chips können auch den Batteriestatus überwachen und Probleme frühzeitig erkennen.
Betrieb einer Lithium-Ionen-Batterie innerhalb ihres Nennspannungsbereichs von 3,7 V trägt dazu bei, seine Lebensdauer zu verlängern. Das Laden über 4,2 V oder das Entladen unter 3,0 V kann die Batterie beschädigen.Sie verringern die Energiekapazität und erhöhen den Innenwiderstand. Batteriemanagementsysteme halten die Spannung innerhalb sicherer Grenzen, was die Lebensdauer der Batterie verlängert und ihre Leistung verbessert. Die Verwendung kompatibler Ladegeräte und das Aufladen innerhalb des empfohlenen Temperaturbereichs tragen ebenfalls zur Batteriesicherheit und Haltbarkeit der Akkus bei.
Die Hersteller entwickeln Geräte, die mit der Standardspannung von 3,7 V arbeiten.. Sie wählen Lithium-Ionen-Akkus mit passender Spannung, Kapazität und Entladestrom. Ein sorgfältiges Design stellt sicher, dass der Akku gleichmäßig Energie liefert und den Strombedarf des Geräts deckt. Eine gleichbleibende Spannung und Energiedichte sorgen für einen reibungslosen und sicheren Betrieb der Geräte. Durch die Einhaltung dieser Verfahren schaffen die Hersteller zuverlässige, wiederaufladbare Produkte, die länger halten und besser funktionieren.
Zukunft der 3,7V-Standardspannung
Neue Chemiestandards
Wissenschaftler entwickeln weiterhin neue Arten von Lithium-Ionen-Batterien. Diese Batterien der nächsten Generation könnten die Standardspannung der heutigen Modelle verändern. Einige Chemikalien, wie Lithiumeisenphosphat, haben eine niedrigere Nennspannung von etwa 3,2 bis 3,3 Volt. Dieser Batterietyp bietet ein gleichmäßiges Spannungsprofil und eignet sich gut für Geräte, die eine konstante Leistung benötigen. Andere Chemietypen, wie Semi-Solid-State- und Solid-State-Batterien, weisen höhere Nennspannungen auf und erreichen bis zu 4,0 Volt pro Zelle. Diese Batterietechnologien der nächsten Generation können mehr Energie speichern und können die Sicherheit verbessern.
| Batteriechemie | Nennspannung (V) | Maximale Spannung (V) | Anmerkungen |
|---|---|---|---|
| Herkömmliche Li-Ion | 3.6 - 3.7 | 4.2 | Standard-Lithium-Ionen-Akku |
| LiFePO4 | ~3.2 - 3.3 | 3.65 | Niedrigere Spannung, stabiler Ausgang |
| Semi-Solid-State | 3.7 - 3.8 | 4.2 - 4.3 | Geringfügig höhere Spannung, neue Technologie |
| Solid-State (Zukunftstechnologie) | ~3.8 - 4.0 | ~4.3 - 4.4 | Höhere Spannung, in Entwicklung |
Diese Veränderungen in der Batteriechemie zeigen, dass die Standardspannung von 3,7 V möglicherweise nicht ewig gilt. Mit dem Erscheinen neuer Designs könnte die Spannung jeder Batteriezelle je nach den verwendeten Materialien steigen oder fallen.
Trends und Innovationen
Viele Trends in der Lithium-Ionen-Batterieforschung konzentrieren sich auf die Verbesserung der Energiedichte, der Sicherheit und der Lebenserwartung. Festkörperbatterien ersetzen flüssige Elektrolyte mit festen. Diese Änderung erhöht die Energiespeicherung und macht die Batterie sicherer. Siliziumanoden können mehr Lithiumionen aufnehmen als Graphit, was die Energiedichte erhöht und die Spannungsnormen beeinflussen kann. Neue Kathodenmaterialien und bessere Zellkonstruktionen tragen ebenfalls dazu bei, dass Batterien mehr Energie speichern und länger halten.
- Spinellzellen mit höherer Spannung, die Mangan verwenden, erreichen etwa 3,8 Volt.. Diese Batterien haben eine höhere Leistung und eine bessere thermische Stabilität. Sie können jedoch eine geringere Kapazität und eine kürzere Lebensdauer haben, wenn sie zu lange mit hohen Spannungen geladen werden.
- Eine niedrigere Ladespannung, z. B. das Anhalten bei 3,92 Volt statt bei 4,2 Volt, kann die Lebensdauer der Batterie verdoppeln, verringert aber die Energiekapazität.
- Mäßige Lade- und Entladeraten tragen dazu bei, die Batterie gesund zu erhalten und den Anstieg des Innenwiderstands zu verlangsamen.
Die Marktentwicklung zeigt, dass die 3,7-V-Lithium-Ionen-Akku wird in der Unterhaltungselektronik beliebt bleiben. Neue Batterietypen mit unterschiedlichen Spannungen werden jedoch zunehmend für Elektrofahrzeuge, Netzspeicher und medizinische Geräte eingesetzt. Festkörperbatterien könnten bald in Produktion gehen und eine doppelt so hohe Energiedichte bieten und mehr Sicherheit.
Die Batterietechnologien der nächsten Generation werden wahrscheinlich mehr Auswahlmöglichkeiten bei Spannung, Energie und Design bieten. Die Forschung wird fortgesetzt und die Lithium-Ionen-Batterie wird sich weiterentwickeln, um den wachsenden Energiebedarf der Welt zu decken.
Die 3,7 V Standard in der Lithium-Ionen-Batterietechnik ergibt sich aus der Chemie von Anode und Kathode. Diese Spannung ermöglicht eine hohe Energiedichte, ein leichtes, wiederaufladbares Batteriedesign und eine lange Zyklusdauer.
- Die meisten Unterhaltungselektronikgeräte und Elektrofahrzeuge verwenden diese Batterie, weil sie Gleichgewicht zwischen Energie, Sicherheit und Leistung.
- Die 3,7V Spannungsbereich gestaltet das Batteriedesign, die Gerätekompatibilität und die Sicherheitsprotokolle.
- Künftige Fortschritte bei den Batterien, wie Solid-State-Batterienkann die Standard-Spannung und Energiespeicherung.
Bei der Auswahl eines wiederaufladbaren Akkus sollten die Nutzer immer auf die Spannungsnorm achten, um eine sichere und effiziente Energienutzung zu gewährleisten.
FAQ
Was bedeutet "Nennspannung" bei Lithium-Ionen-Batterien?
Die Nennspannung gibt die durchschnittliche Spannung an, die eine Batterie bei normalem Gebrauch liefert. Sie hilft Ingenieuren bei der Entwicklung von Geräten, die sicher und effizient mit der Batterie arbeiten.
Warum haben manche Lithiumbatterien unterschiedliche Spannungen?
Die Batteriespannung hängt von den verwendeten Materialien ab. Lithium-Eisen-Phosphat-Batterien beispielsweise verwenden andere Chemikalien als normale Lithium-Ionen-Batterien. Durch diese Veränderung haben sie eine niedrigere Spannung.
Kann ein Gerät eine Batterie mit einer anderen Spannung verwenden?
Geräte benötigen Batterien mit der richtigen Spannung. Die Verwendung einer falschen Spannung kann das Gerät beschädigen oder dazu führen, dass es schlecht funktioniert. Schauen Sie immer im Handbuch des Geräts nach, bevor Sie den Batterietyp wechseln.
Wie wirkt sich der 3,7-V-Standard auf die Lebensdauer der Batterien aus?
Der 3,7-V-Standard sorgt für ein Gleichgewicht zwischen Energiespeicherung und Sicherheit. Er ermöglicht eine längere Lebensdauer der Batterie und eine gleichmäßige Energieversorgung. Das Aufladen oder Entladen außerhalb der sicheren Spannungsgrenzen kann die Lebensdauer der Batterie verkürzen.
Sind Batterien mit höherer Spannung immer besser?
| Höhere Spannung | Profis | Nachteile |
|---|---|---|
| Ja | Mehr gespeicherte Energie | Kann die Sicherheit verringern |
| Nein | Nicht immer sicherer | Kann die Lebenserwartung verkürzen |
Eine höhere Spannung kann mehr Energie speichern, kann aber die Sicherheit und die Lebensdauer der Batterie verringern.

