Selecting a power source for medical devices is a critical decision. Your choice directly impacts patient safety and the reliability of your devices. With the lithium battery market for medical equipment projected to grow at a 9.5% CAGR, getting it right is essential. Battery failures are a significant cause of recalls for medical devices.
To successfully choose lithium batteries, you need a structured approach. This framework balances three pillars. You must define technical specifications for lithium-ion batteries. You need to confirm regulatory compliance like IEC 62133. You should also vet manufacturer quality with ISO 13485.
How to Choose Lithium Batteries: Key Specs

Your first step is to define the technical specifications your medical device requires. A mismatch here can lead to poor performance or outright failure. You must analyze your device’s power profile to align it with the right battery technology.
Match Chemistry to Device Needs
The chemistry of a battery determines its core performance characteristics. You need to choose a chemistry that aligns with your device’s function, whether it’s a single-use surgical tool or a long-term implantable. The right choice balances energy, power, lifespan, and safety.
Choosing Chemistries: Lithium-Ion Batteries vs. Others
You have several lithium chemistries to consider, each with distinct advantages.
For Non-Rechargeable Devices: Primary (non-rechargeable) lithium cells are ideal for devices requiring long-term, reliable power without the need for charging. Two common choices are Lithium Manganese Dioxide (Li-MnO2) and Lithium Thionyl Chloride (Li-SOCl2). Li-MnO2 batteries are often used in pacemakers and defibrillators. Li-SOCl2 batteries are excellent for medical implants due to their very long life.
Profi-Tipp: Li-MnO2 delivers high-power pulses for devices needing short bursts of energy. Li-SOCl2 provides a lower, continuous current for long-duration applications.
| Merkmal | Li-MnO2 | Li-SOCl2 |
|---|---|---|
| Nennspannung | 3.0V | 3.6V |
| Discharge Current | High pulse | Continuous, low to moderate |
| Die Energiedichte | Hoch | Very high |
| Self-Discharge Rate | Niedrig | Extremely low |
| Preis | Cheaper | More expensive |
| Typical Medical Applications | Pacemakers, defibrillators | Medical implants |
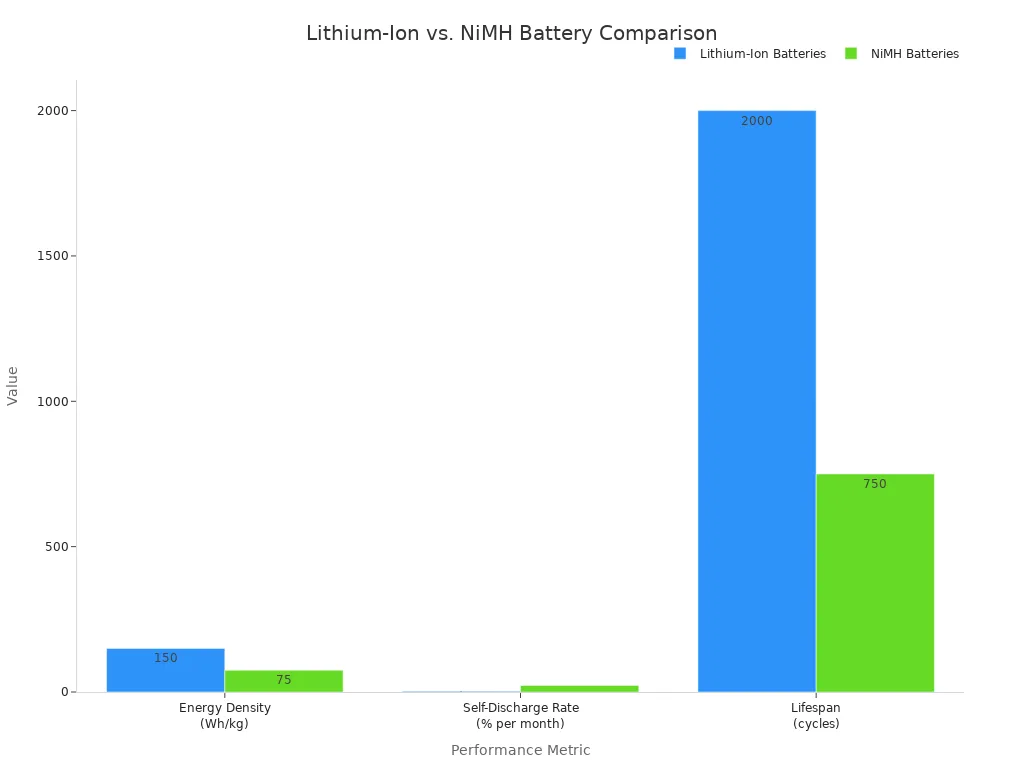
For Rechargeable Devices: Rechargeable lithium-ion batteries are the standard for many portable medical devices, from infusion pumps to patient monitors. They offer significant advantages over older technologies like Nickel-Metal Hydride (NiMH). Lithium-ion batteries are lighter, charge faster, and last longer. They also do not suffer from the “memory effect” that plagues NiMH cells.
For mobile medical equipment like carts on wheels, Lithium Iron Phosphate (LFP or LiFePO4) is an excellent choice. Hospitals often select LFP batteries for these devices because of their superior thermal safety and extremely long cycle life. This longevity reduces the total cost of ownership. Compared to Sealed Lead-Acid (SLA) batteries, the difference is stark.
- Sealed Lead-Acid (SLA): ~300 cycles
- Lithium Cobalt Oxide (LiCoO2): Up to 1,000 cycles
- Lithium Iron Phosphate (LiFePO4): Over 2,000 cycles
Diese extended cycle life means you will replace batteries far less often, a key benefit in a busy medical environment. Your decision to choose lithium batteries for these applications improves both reliability and operational efficiency.
Calculate Energy Density and Capacity
Next, you must calculate the energy your device needs. Capacity, measured in milliamp-hours (mAh) or amp-hours (Ah), tells you how much charge the battery can hold. Energy density tells you how much energy is packed into a certain size or weight.
To ensure your device runs for the required duration, you can use a simple formula.
- Find Battery Energy (Wh):
Energy (Wh) = Capacity (Ah) × Voltage (V)- Find Device Power (W): Check your device’s power consumption in watts.
- Calculate Runtime:
Runtime (hours) = Energy (Wh) / Power (W)For example, a battery with 4000mAh (4Ah) capacity and 11.1V has 44.4Wh of energy. If your device consumes 2.22W, the theoretical runtime is 20 hours (
44.4Wh / 2.22W). Always account for efficiency losses, which can reduce runtime by 10-30%.
The form factor also matters. Common cylindrical cells like the 18650 are robust and widely available. Lithium polymer (LiPo) packs offer more flexibility in shape and size, allowing you to fit more capacity into custom-designed devices.
Determine Voltage and Discharge Rate
You must match the battery’s voltage to your device’s operating requirements. Most lithium-ion cells, including the popular 18650 and 21700 models, have a nominal voltage of 3.6V to 3.7V. You can connect cells in series to increase the total voltage of the battery pack.
Discharge rate, or C-rate, is equally important. It defines how quickly the battery can safely release its energy.
- High-power devices, like surgical tools, need a high C-rate for bursts of energy.
- Geräte mit niedrigem Stromverbrauch, like ambient sensors, require a low C-rate for long, steady operation.
Choosing the wrong C-rate can damage the battery or cause the device to underperform. This is a critical step when you choose lithium batteries.
Assess Operating Temperature Range
Finally, you must consider the environment where your medical devices will operate. Temperature significantly affects battery performance, safety, and lifespan. Standard lithium batteries operate well between -20°C and 60°C.

- Extreme Heat: High temperatures can increase internal pressure, posing a safety risk. It also accelerates degradation and reduces the battery’s overall life.
- Extreme Cold: Low temperatures increase internal resistance, which reduces the available capacity and power. Implantable medical devices may require special low-temperature chemistries like Li-SOCl₂ that function reliably down to -40°C or even lower.
A Note on Sterilization: High-temperature sterilization methods like autoclaving can permanently damage lithium battery packs. You should explore alternative methods, such as low-temperature chemical cleaning or peroxide-gas systems, to ensure the integrity of your power source. Your final decision to choose lithium batteries must account for these post-manufacturing processes.
Safety and Compliance for Medical Devices

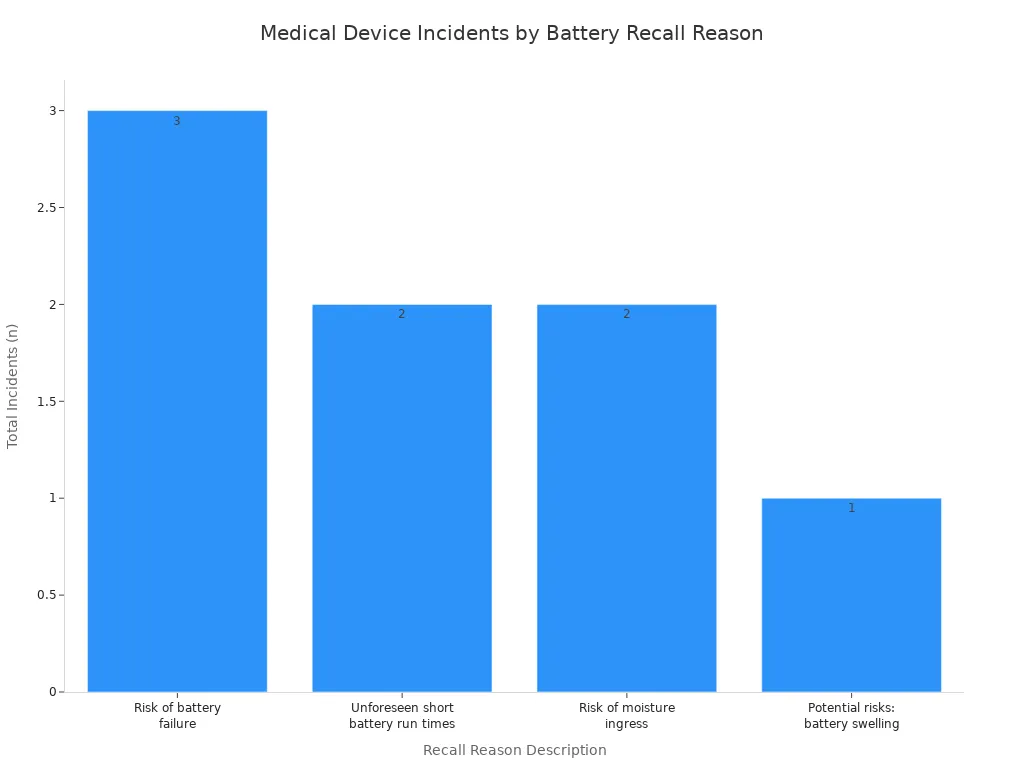
Beyond technical performance, you must prioritize safety and regulatory compliance. Failing to meet these standards can lead to device recalls, legal liabilities, and severe patient harm. The FDA tracks incidents related to battery hazards, which often stem from poor design or manufacturing. These failures can result in dangerous events like explosions, fires, or overheating, leading to thermal runaway. Your adherence to established standards is the best defense against these risks.
Verify Key Safety Certifications (IEC 62133)
You must ensure your batteries are certified to IEC 62133. This is the primary global standard for the safety of rechargeable lithium batteries. The FDA recognizes these standards as a key part of ensuring medical device safety. For example, IEC 62133 is cited in over 13% of reports for FDA-cleared home sleep apnea devices. The standard mandates rigorous testing to prevent thermal runaway and other failures.
Key IEC 62133-2 Tests Include:
- External short circuit tests at elevated temperatures (+55°C).
- Thermal abuse tests to simulate overheating conditions.
- Crush and forced discharge tests to assess mechanical and electrical stress.
- Vibration and shock tests to ensure durability.
These stringent safety protocols are designed to address common causes of battery failure and are essential for battery safety.
Confirm UN/DOT 38.3 Transport Compliance
You need to ship your medical devices globally. Therefore, your batteries must comply with UN/DOT 38.3. This standard ensures the safe transportation of lithium batteries by air, sea, or ground. Compliance is mandatory. You must obtain proper documentation from your supplier to prove it.
Required documents typically include:
- A complete UN 38.3 test report.
- A test summary that references the UN Manual of Tests and Criteria.
Without this, your devices can be grounded, causing significant shipping delays.
Ensure Biocompatibility (ISO 10993)
For medical devices that contact the body, you must verify biocompatibility. ISO 10993 is the key set of standards for this evaluation. The battery’s casing and any external components must not cause adverse reactions. The FDA requires this testing for implantable and skin-contact devices. For permanent implants, the safety considerations are extensive.
| Biological Effect (Permanent Contact) | Required Test |
|---|---|
| Cytotoxicity & Genotoxicity | ✔ |
| Sensitization & Irritation | ✔ |
| Systemic Toxicity & Pyrogenicity | ✔ |
| Implantation & Carcinogenicity | ✔ |
Meeting these medical standards is a critical step in your FDA submission and ensures patient safety.
Selecting a Qualified Manufacturer
Your choice of a battery manufacturer is as critical as the battery’s specifications. A qualified partner ensures quality, reliability, and compliance for your medical devices. Medical device manufacturers must perform rigorous due diligence to mitigate risks.
Prioritize ISO 13485 Certified Partners
You should partner with manufacturers certified to ISO 13485. This standard is the quality management system for medical devices. It goes far beyond the general ISO 9001 standard. ISO 13485 requires a much stronger focus on risk management and regulatory compliance.
Why ISO 13485 Matters:
- Regulatory Focus: It prioritizes meeting regulatory requirements over general customer satisfaction.
- Stricter Documentation: It demands detailed documentation that must be retained for the lifetime of the device.
- Risk Management: It integrates risk management throughout the entire product lifecycle.
Working with an ISO 13485 certified partner shows their commitment to the quality and safety standards that medical device manufacturers must uphold.
Evaluate Design and Testing Capabilities
You need a partner with robust engineering and testing capabilities. Standard specification sheets are not enough. Your supplier must prove the battery performs reliably in your specific application. Ask potential medical device manufacturers about their in-house testing services. They should be able to perform a wide range of tests.
- Performance Tests: Cycle life, storage, and discharge rate tests.
- Safety Tests: Short circuit, overcharge, crush, and thermal abuse tests.
- Environmental Tests: Altitude simulation and high/low temperature tests.
Top-tier medical device manufacturers also offer custom design services for unique devices, such as surgical tools or ventilators, ensuring the power solution is perfectly tailored.
Assess Supply Chain Stability
You must assess your supplier’s supply chain. The market for battery raw materials like lithium and cobalt is volatile. A high concentration of material processing is located in China, creating potential vulnerabilities. You should ask about their strategies to ensure a stable supply. A transparent supply chain is essential for medical device manufacturers. It helps you avoid production delays and ensures long-term availability for your critical devices.
Your final decision to choose lithium batteries for medical devices requires a structured approach. This ensures both performance and patient safety. Use this checklist to guide your selection of lithium-ion batteries.
- Define Technical Needs: Match the lithium chemistry, capacity, and voltage to your device’s power profile.
- Verify All Certifications: Confirm the battery meets key safety standards like IEC 62133 and UN 38.3 for transport.
- Select a Qualified Partner: Vet your manufacturer for ISO 13485 certification to guarantee quality and risk management.
Following these steps ensures your devices have a reliable and compliant power source, prioritizing safety.
FAQ
What is the main difference between ISO 13485 and ISO 9001?
ISO 9001 focuses on general quality and customer satisfaction. You should prioritize ISO 13485 because it is a stricter standard. It specifically requires comprehensive risk management and regulatory compliance for medical devices, ensuring a higher level of safety and documentation.
Why is IEC 62133 certification so important?
You must verify IEC 62133 certification for battery safety. This global standard mandates rigorous tests for short circuits, overheating, and physical damage. It is your best defense against battery failures like thermal runaway, which protects both patients and your device’s integrity.
Can you sterilize lithium batteries with heat?
No, you should never use high-heat sterilization like autoclaving. Heat permanently damages lithium batteries and creates serious safety risks. You must use low-temperature methods, such as chemical or gas sterilization, to clean devices with integrated batteries.
When should you consider a custom battery pack?
You should consider a custom pack for devices with unique shapes or specific power demands. Standard cells may not fit or perform correctly. A custom solution ensures the battery is perfectly optimized for your device’s size, runtime, and discharge requirements.

