
People rely on lithium ion battery technology every day, from mobile phones to electric vehicles. The rapid rise of electric cars and the growing demand for portable electronics have pushed battery sales to new heights. Over the last decade, the global lithium-ion batteries market has expanded quickly, with the electric vehicle (EV) sector showing the fastest growth. Chemistry and engineering improvements have made batteries safer and more efficient, helping EV adoption soar and powering a wide range of electric devices.
Battery costs have dropped by 99% over 30 years, while energy density has increased fivefold, making electric mobility possible for millions.
Reliability and Safety
Key Factors
Lithium ion battery reliability and battery safety depend on several important factors. Scientists and engineers study these factors to make batteries safer and longer-lasting. The table below shows the main factors that affect reliability and safety, along with their impacts and underlying mechanisms:
| Primary Factor | Impact on Reliability and Safety | Underlying Mechanisms and Effects |
|---|---|---|
| Temperature | High temperatures speed up aging, damage the battery’s protective layers, and lower thermal stability. Low temperatures reduce battery capacity and charging efficiency. Rapid temperature changes make batteries wear out faster. | Chemical reactions happen faster at high temperatures, breaking down battery materials. At low temperatures, lithium ions move more slowly, which reduces performance. |
| Vibration | Vibration can crack battery parts and cause materials inside to break loose. This increases resistance and makes the battery less stable. | Shaking or bumping the battery causes tiny cracks and uneven movement of lithium ions, which can damage the battery over time. |
| Charging/Discharging Rates | Charging or discharging too quickly can cause dangerous lithium buildup, overheating, and even fire. Fast rates also wear out the battery faster. | Quick charging can create sharp lithium spikes inside the battery, which may lead to short circuits and safety risks. |
Tip: Keeping batteries at moderate temperatures and charging them at recommended rates helps improve both reliability and safety.
Environmental conditions also play a big role. High temperatures and humidity, especially in places near the ocean or during storms, can make batteries age faster and increase the risk of thermal runaway. Water getting inside the battery can cause fires, as seen during flooding events. Good design, strong seals, and regular maintenance help prevent these problems. Manufacturers use safety testing to check how batteries perform in different environments. They also use safety testing to make sure batteries can handle heat, moisture, and vibration before they reach consumers.
Real-World Importance
Battery safety and reliability matter in everyday life and across many industries. When batteries fail, the results can be serious. Several well-known incidents show why safety testing and quality control are so important:
| Incident/Event | Description | Impact on Users/Industries |
|---|---|---|
| 2006 Lithium-ion Battery Recall | Nearly six million batteries were recalled after fires and injuries caused by internal short circuits. These batteries had passed safety testing but failed during normal use. | Millions of consumers and manufacturers were affected by the recall. |
| Hoverboard Fires | Many hoverboards caught fire due to low-quality batteries. Certification and better safety testing reduced these incidents. | Safety concerns led to new rules and better battery safety standards. |
| Samsung Galaxy Note 7 Battery Fires | Manufacturing problems caused battery fires, leading to a global recall and the phone’s discontinuation. | Samsung lost money and trust. The industry focused more on battery safety and reliability. |
| Boeing 787 Dreamliner Battery Issues | Battery defects led to safety concerns and grounded planes. The issues were fixed after more testing and design changes. | Airlines faced delays, and the aviation industry increased battery safety checks. |
| 2018 WestJet Plane Incident | A burning e-cigarette battery forced a plane to return to the airport. | This event highlighted the need for strict rules on battery transport and safety. |
| Electric Vehicle Fire Statistics | Electric vehicles have fewer fires per billion kilometers than gasoline cars. For example, Tesla reports 2 fires per billion kilometers, while gasoline cars have 90. | This shows that battery safety has improved, but ongoing safety testing and monitoring remain important. |
Battery failures, though rare, can cause fires or other dangerous events. These incidents show the need for early fault detection and real-time monitoring. Companies now use advanced diagnostics and big data to predict problems before they happen. Safety testing and regular maintenance help prevent failures and keep users safe.
Note: Battery safety is not just about preventing fires. It also means making sure batteries last longer, work well in different environments, and do not fail unexpectedly. Regular safety testing and careful design help achieve these goals.
Lithium Ion Battery Chemistry
Structure and Components
A lithium ion battery contains several important parts that work together to store and release energy. Each part has a special job:
- Anode: This negative electrode, usually made from graphite, stores lithium ions during charging and releases them during use.
- Cathode: The positive electrode uses metal oxides like lithium cobalt oxide. It sets the battery’s voltage and capacity by holding or releasing lithium ions.
- Electrolyte: This liquid, gel, or solid allows lithium ions to move between the anode and cathode but blocks electrons, helping the battery work safely.
- Separator: A thin, porous layer keeps the anode and cathode apart to prevent short circuits, while still letting lithium ions pass through.
- Current Collectors: Metal sheets (aluminum for the cathode, copper for the anode) carry electrons between the electrodes and the device.
- Casing: A strong outer shell protects the inside parts and keeps the battery safe.
The separator’s design affects both safety and performance. Foam separators with even pores help lithium ions move smoothly and keep the battery cool during fast charging.
Chemical Reactions
Lithium-ion batteries store and release energy by moving lithium ions between the electrodes. The process works like this:
- During charging, lithium ions travel from the cathode to the anode, where they stay until the battery is used.
- When the battery powers a device, lithium ions move back to the cathode, releasing electrical energy.
- This back-and-forth movement changes chemical energy into electrical energy and lets the battery recharge many times.
Some advanced batteries, like lithium-air types, use special reactions that can store even more energy. These reactions use solid-state electrolytes and special catalysts to improve how well the battery works and how long it lasts.
Types of Chemistries
Different lithium-ion batteries use different materials for their electrodes. Each type has unique strengths and weaknesses. The table below shows the most common chemistries and their main features:
| Chemistry | Cathode Composition | Specific Energy | Safety | Lifespan | Cost | Typical Applications |
|---|---|---|---|---|---|---|
| LCO (Lithium Cobalt Oxide) | Cobalt oxide | High | Low | Short | High | Mobile devices (phones, laptops) |
| NMC (Lithium Nickel Manganese Cobalt Oxide) | Nickel, Manganese, Cobalt | High | Moderate | Moderate to High | Moderate | Electric vehicles, energy storage |
| NCA (Lithium Nickel Cobalt Aluminum Oxide) | Nickel, Cobalt, Aluminum | High | Lower than NMC | Moderate | High | High-performance EVs |
| LFP (Lithium Iron Phosphate) | Iron, Phosphate | Lower | High | Long | Low | Energy storage, short-range EVs |
| LMO (Lithium Manganese Oxide) | Manganese oxide | Moderate | High | Moderate | Moderate | Power tools, blended in EVs for high current |
| LTO (Lithium Titanate) | Lithium titanate anode | Low | Excellent | Very Long | High | Heavy-duty applications, fast charging |
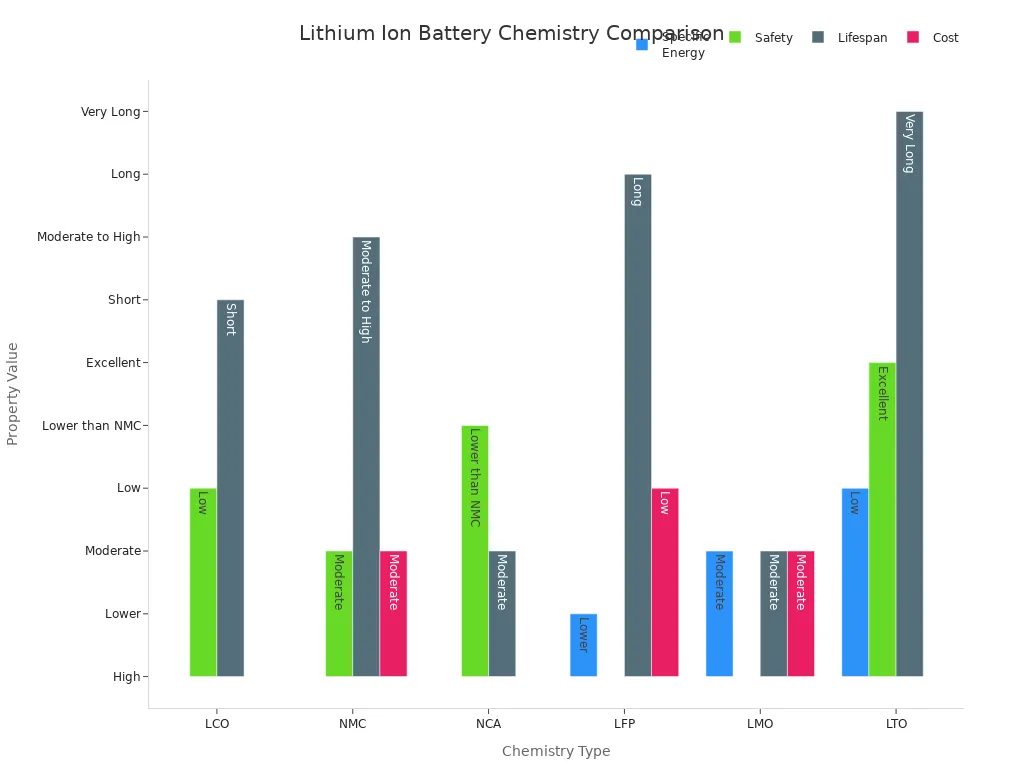
- NMC batteries offer high energy and good safety, making them popular in electric vehicles.
- NCA batteries provide even more energy but cost more and have lower safety.
- LFP batteries last longer and are safer, but store less energy.
- LCO batteries power most phones and laptops because of their high energy, but they do not last as long.
- LMO and LTO batteries focus on safety and fast charging, often used in power tools or heavy-duty vehicles.
Performance of Lithium Ion Battery Technology
Energy Density
Energy density measures how much energy a battery can store for its weight. Lithium-ion batteries stand out for their high energy density compared to other rechargeable battery types. This feature allows electric vehicles (EVs) to travel farther on a single charge and makes portable devices lighter and more powerful. The table below compares the energy density of different battery technologies:
| Battery Type | Typical Energy Density Range (Wh/kg) |
|---|---|
| Lead Acid | 30 – 50 |
| Nickel-Cadmium (NiCd) | 45 – 80 |
| Nickel-Metal Hydride (NiMH) | 60 – 120 |
| Lithium-Ion (overall) | 50 – 260 |
| Lithium-Ion Subtypes | Energy Density Range (Wh/kg) |
|---|---|
| Lithium Titanate (LTO) | 50 – 80 |
| Lithium Cobalt Oxide (LCO) | 150 – 200 |
| Lithium Nickel Manganese Cobalt Oxide (NMC) | 150 – 220 |
| Lithium Iron Phosphate (LFP) | 90 – 160 |
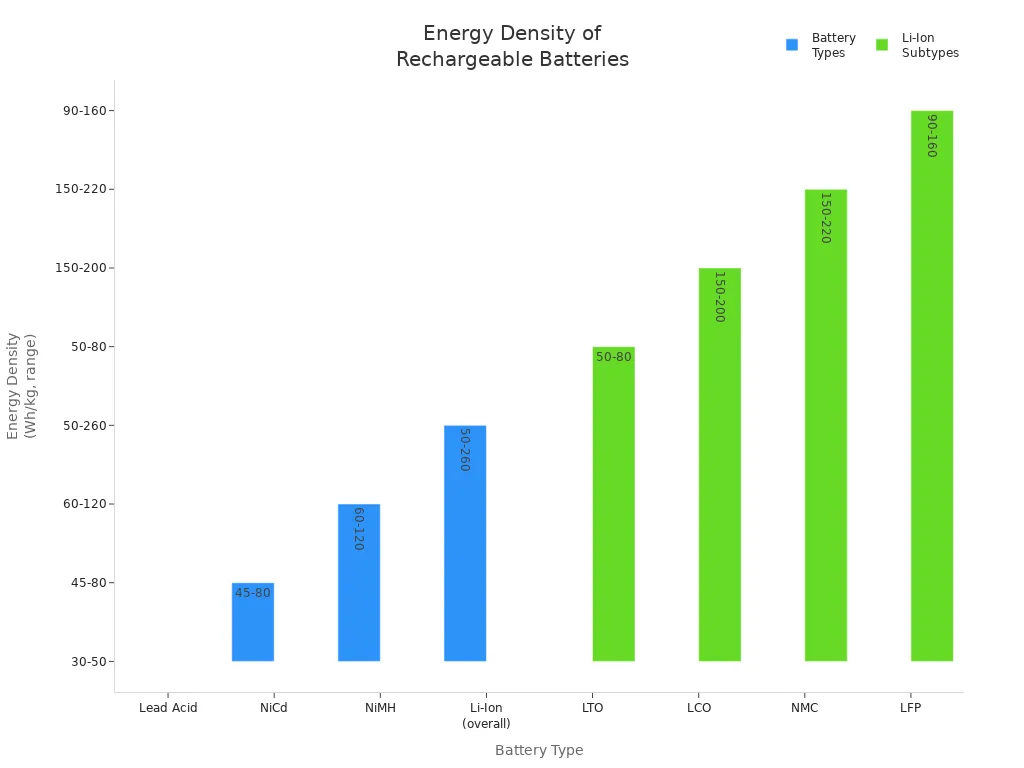
NMC and LCO chemistries offer the highest energy densities, which helps EVs achieve longer ranges and better performance benefits.
Lifespan and Cycle Life
The lifespan of lithium-ion batteries depends on how many times they can charge and discharge before losing capacity. This is called cycle life. Lithium ion battery technology gives EVs and electric devices a much longer cycle life than older battery types. For example:
| Battery Type | Typical Cycle Life (cycles) | Efficiency (%) | Notes on Performance and Usage |
|---|---|---|---|
| Lithium-ion | 3,000 to 5,000+ | 95–98 | Longest cycle life; BMS integrated for safety and performance |
| Nickel-Metal Hydride (NiMH) | 500 to 800 | 70–80 | Moderate cycle life; less cost-effective long-term |
| Lead-acid | 200 to 800 | 70–85 | Shortest cycle life; faster degradation under heavy use |
- Lithium iron phosphate (LFP) batteries can last between 2,000 and 5,000 cycles, making them ideal for EVs and energy storage.
- Lithium-ion batteries store more energy and last longer than NiMH batteries.
- Lead-acid batteries degrade quickly, especially in electric vehicles.
Many factors affect battery lifespan. High charge currents, high state of charge, and extreme temperatures can speed up aging. Overcharging and deep cycling also reduce performance. Scientists have found that changes inside the battery, such as growth of the SEI layer and electrolyte drying, cause capacity loss over time. Good charging habits and temperature control help extend battery life in both consumer and industrial technology.
Charging and Efficiency
Charging efficiency shows how well a battery stores the energy it receives. Lithium-ion batteries have an average charging efficiency of about 96.4%. This means almost all the energy used to charge the battery is stored and available for use. High efficiency reduces energy waste and lowers electricity costs for EV owners and electric device users. Charging rate, temperature, and state of charge can affect efficiency. For example, charging too quickly or at very high or low temperatures can lower performance and shorten battery life. Improving efficiency in lithium ion battery technology helps reduce emissions and saves money, especially as more people use electric vehicles.
Tip: Charging at moderate speeds and keeping batteries cool can help maintain high performance and extend battery lifespan.
Battery Safety Features

Modern lithium ion batteries rely on several safety features to prevent overheating, short circuits, and thermal runaway. These reliable safety features help protect users from fire and other hazards, making battery safety a top priority for manufacturers.
Protection Circuitry
Protection circuitry acts as the first line of defense in battery safety. These systems monitor each cell’s voltage, current, and temperature. When the battery management system detects abnormal conditions, it takes action to stop dangerous events. The following table shows how these safety features work:
| Protection Mechanism | Description |
|---|---|
| Overcharge Protection | Monitors voltage and disconnects charging when limits are reached to prevent overcharge and runaway. |
| Overdischarge Protection | Disconnects the load if voltage drops too low, avoiding battery damage. |
| Overcurrent Protection | Interrupts current flow if it becomes too high, protecting both battery and devices. |
| Short Circuit Protection | Quickly disconnects the battery to prevent rapid discharge and heat buildup. |
| Temperature Monitoring | Reduces charging current or disconnects the battery to prevent overheating or freezing. |
| Cell Balancing | Keeps all cells at equal voltage, reducing stress and the risk of runaway. |
These safety features follow strict international standards, ensuring external battery safety designs meet global requirements.
Separators
Separators play a key role in battery safety by keeping the anode and cathode apart. Most separators use polyethylene or polypropylene. Polyethylene melts at about 135°C, closing its pores and stopping ion flow if the battery gets too hot. Polypropylene has a higher melting point and keeps its shape, delaying thermal runaway. Some separators use both materials in layers, while others add ceramic coatings for extra strength. New functional separators use special coatings to control ion movement and improve safety. These materials and designs help prevent short circuits and fire.
Battery Management Systems
A battery management system (BMS) uses advanced sensors and software to watch over the battery in real time. The BMS checks voltage, current, and temperature, and can predict problems before they happen. Some systems use fiber sensors to detect changes inside the battery, giving early warnings before runaway starts. The BMS also estimates how much charge the battery has left and how healthy it is. These safety features help keep batteries safe, reliable, and long-lasting.
Tip: Safety features in lithium ion batteries continue to improve as engineers develop better materials and smarter systems.
Risks and Mitigation
Overheating
Overheating is a major safety concern in lithium ion batteries. Several factors can cause batteries to get too hot and lead to fire or even explosion. Recent research highlights the most common causes:
- Mechanical abuse, such as crushing or puncturing, can damage the battery and cause internal short circuits.
- Electrical abuse, like overcharging or overdischarging, generates heat and speeds up battery wear.
- Battery chemistry matters. NMC batteries are more likely to experience thermal runaway than LFP batteries.
- Confined storage spaces increase the risk of fire if overheating occurs.
| Cause Type | Description | Key Details |
|---|---|---|
| Mechanical Abuse | Physical damage (puncture, crushing) causing internal short circuits | High state of charge increases risk; temperatures above 233°C can trigger explosion or fire |
| Electrical Abuse | Overcharging or overdischarging beyond safe voltage limits | Overcharge tolerance depends on battery chemistry; packaging affects thermal behavior |
| Thermal Abuse | Poor heat dissipation or faulty management systems | Localized heating leads to capacity loss, fire, or explosion; separator melts at high temps |
To reduce overheating, experts recommend several safety measures:
- Use cooling systems to manage heat.
- Train workers to handle batteries safely.
- Set up emergency shutdown procedures to stop power if a problem occurs.
Tip: Watch for signs of thermal runaway, such as bulging, popping sounds, or strange odors. These signs mean the battery could catch fire or explode.
Short Circuits
Short circuits are another serious risk for battery safety. They can happen when the battery is damaged or used incorrectly. Overdischarge can cause copper inside the battery to move and create an internal short, even without outside damage. Mechanical abuse, like bending or crushing, also increases the chance of a short circuit. Thicker electrodes and higher energy density make batteries more likely to short and overheat. Studies show that about 56% of thermal runaway events in electric vehicles come from internal short circuits. This can lead to fire or explosion.
To prevent short circuits, manufacturers use several design features:
- Stringent sealing and careful material selection.
- High-quality separators with strong thermal stability.
- Robust shells and electrical insulation.
- Battery management systems to detect problems early.
- Safe charging practices and proper handling during transport.
A new design uses a thin tin layer on the lithium surface. This layer stops dendrites from forming, which helps prevent internal shorts and improves battery safety.
Safe Design
Safe design is key to battery safety and reducing the risk of explosion or fire. Manufacturers follow strict safety rules and use advanced tools to spot hazards. They keep humidity very low in battery factories to stop moisture from causing problems. They also control the battery’s state of charge during production to lower the risk of runaway. Fire protection systems, like sprinklers and heat-seeking water cannons, help stop fires quickly.
Key safety principles include:
- Using protection circuitry to monitor voltage, current, and temperature.
- Installing porous separators that melt and shut down the battery if it gets too hot.
- Deploying battery management systems to watch for overheating and other dangers.
- Training workers and following global safety standards.
Note: Good design, regular maintenance, and proper handling all work together to keep lithium ion batteries safe and reliable.
Advances in Lithium Ion Battery Technology
New Materials
Researchers have introduced several new materials to improve lithium ion battery performance and safety. These materials help electric vehicles (EVs) and other electric devices last longer and charge faster.
- Silicon anodes increase energy density by up to 50% compared to graphite. They allow EVs to travel farther on a single charge. Engineers use silicon-carbon composites and nanostructured silicon to solve problems with swelling during charging.
- Lithium metal anodes offer even higher energy density and can lower costs. Some new designs use anode-free setups, but engineers still work to improve efficiency and lifespan.
- Solid-state electrolytes replace flammable liquids with solid materials. This change boosts safety and energy density. Companies expect to release solid-state batteries for electric cars around 2027.
- Semi-solid-state electrolytes combine liquid and polymer materials. They balance safety with easier manufacturing.
- New cathode materials, such as lithium manganese iron phosphate (LMFP), provide higher energy density than traditional LFP cathodes. These materials keep costs low and improve safety.
These advances help batteries store more energy, last longer, and stay safer during use.
Enhanced Safety Protocols
Manufacturers have changed how they make and handle lithium ion batteries to meet higher safety standards. The table below shows how safety protocols affect each stage of a battery’s life:
| Lifecycle Stage | Impact of Enhanced Safety Protocols |
|---|---|
| Material Sourcing | Safer and more careful handling of hazardous materials increases cost and complexity. |
| Manufacturing | Factories use clean rooms and strict controls to prevent accidents and improve safety. |
| Product Certification | Batteries go through more tests at every level to reduce fire risks. |
| Transportation & Warehousing | Strict rules for shipping and storing batteries raise insurance and shipping costs. |
| Installation & Operation | Fire suppression systems and thermal management protect electric vehicles and storage sites. |
| End-of-Life Handling | Safe disposal and recycling prevent fires and protect the environment. |
| Regulatory Pressure | New rules, like the EU Batteries Regulation, require better safety and sustainability throughout the battery’s life. |
Manufacturers also train workers to spot damage, use approved chargers, and follow emergency plans. These steps help prevent fires and keep electric devices and EVs safe.
Industry Standards
Industry experts have created strict safety standards for lithium ion batteries. These standards help protect users and guide manufacturers.
- IEC 62133 covers safety for rechargeable batteries in portable electronics. It checks for hazards like overcharging and short circuits.
- UN/DOT 38.3 sets rules for shipping batteries safely, including tests for vibration and impact.
- IEC 62619 applies to batteries in stationary and motive uses, such as backup power and forklifts.
- UL 1642 sets safety standards for battery cells in electronic products.
- UL 2580 focuses on batteries in electric vehicles, testing for real-world dangers like crushing and short circuits.
Regulations push companies to make safer, more reliable batteries. New rules, such as the EU’s battery regulation, require companies to track carbon footprints and use recycled materials. These standards encourage innovation while making sure electric vehicles and other devices meet high safety levels.
Note: Meeting safety standards can increase costs and slow down new technology, but these rules protect people and the environment.
Handling and Storage
Best Practices
Proper handling and storage of lithium ion batteries help prevent accidents and extend battery life. People can follow these best practices to keep batteries safe and reliable:
- Store batteries at a partial charge, around 40-60%, to reduce stress on the cells.
- Place batteries in cool, dry, and well-ventilated areas. The best temperature range is 50°F to 77°F (10°C to 30°C).
- Avoid overcharging or deep discharging. These actions can damage the battery and increase fire risk.
- Use only chargers approved by the manufacturer. Chargers must match the battery’s voltage and capacity.
- Inspect batteries often, especially during long-term storage. Look for damage, swelling, or leaks.
- Store batteries in non-conductive or fireproof containers. This reduces the chance of short circuits and fire.
- Keep batteries away from sunlight, extreme temperatures, and physical harm.
- Prepare for leaks by using drip trays or bunded cabinets. Handlers should wear personal protective equipment.
- Recharge batteries every three months during long-term storage. This prevents self-discharge and keeps cells healthy.
- Never charge batteries on flammable surfaces. Use non-combustible areas instead.
- Dispose of damaged or swollen batteries through proper recycling or hazardous waste programs.
- Store batteries off the floor on racks that allow airflow. Avoid metal surfaces to prevent short circuits.
- In cold weather, use insulated enclosures or heaters to protect batteries.
- In emergencies, move overheating or leaking batteries to a safe surface and contact local waste management for disposal.
Tip: Safe storage and regular checks help prevent fires and support responsible recycling.
Warning Signs
Recognizing early warning signs can prevent battery failures and safety hazards. People should watch for these signs:
- Swelling or bulging of the battery pack
- Leaks or visible moisture
- Unusual odors, such as burning or chemical smells
- Excessive heat during use or charging
- Cracked, deformed, or discolored cases
- Smoke, hissing, or popping sounds
- Rapid loss of charge or failure to power on
If any of these warning signs appear, stop using the battery right away. Move it away from flammable materials and arrange for safe recycling. Never store or charge a battery that shows damage or abnormal behavior. Early action protects people and the environment.
Note: Proper recycling of lithium ion batteries keeps harmful chemicals out of landfills and supports the recovery of valuable materials. Many communities offer special recycling programs for batteries. Always follow local guidelines for safe disposal and recycling.
Understanding lithium ion battery chemistry helps consumers choose the right battery for safety and performance. The table below compares key chemistries:
| Chemistry | Safety | Energy Density | Lifespan (Cycles) |
|---|---|---|---|
| LFP | Excellent | Lower | 2,000–5,000+ |
| NMC | Moderate | Moderate | 1,000–2,000 |
| NCA | Lower | High | ~500 |
| LCO | Limited | High | 500–1,000 |
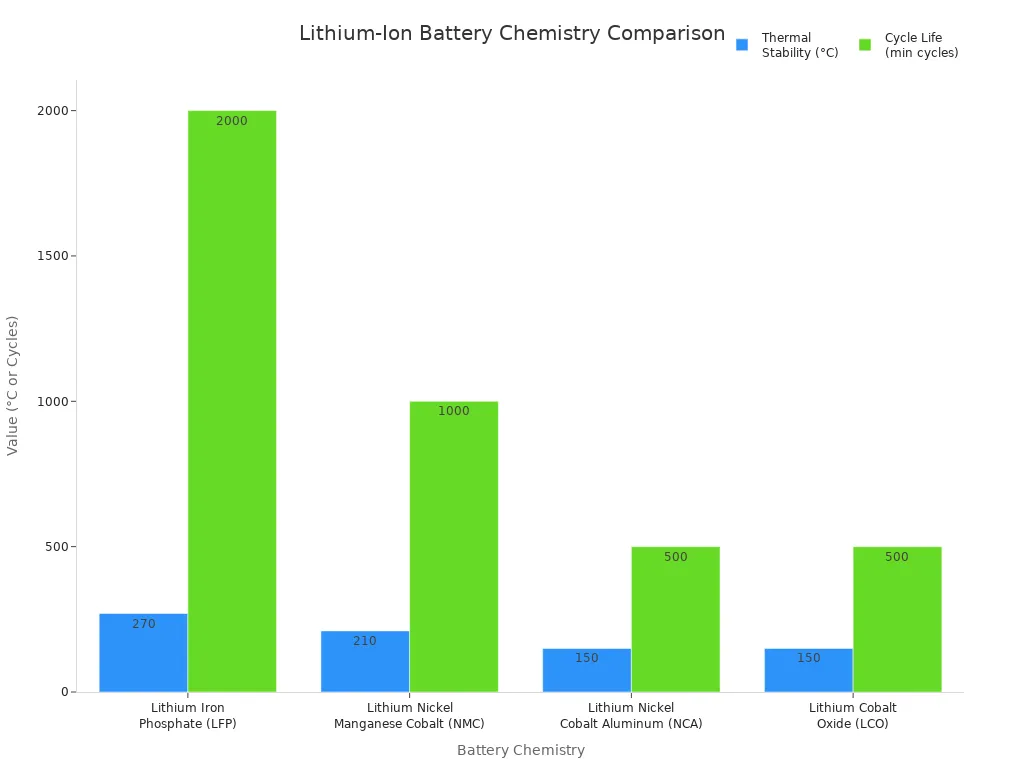
Ongoing innovation, such as solid-state batteries and silicon anodes, improves safety and reliability. For safe use, people should:
- Store batteries in cool, dry places.
- Use approved chargers.
- Inspect batteries regularly for damage.
Smart choices and good habits help everyone stay safe and get the most from lithium ion batteries.
FAQ
What makes lithium ion batteries safer than older battery types?
Lithium ion batteries use advanced separators, protection circuits, and battery management systems. These features help prevent overheating, short circuits, and fires. Manufacturers test batteries for safety before selling them.
How long do lithium ion batteries usually last?
Most lithium ion batteries last between 3 and 5 years. The cycle life ranges from 1,000 to 5,000 charges, depending on the battery type and how people use and store them.
Can people recycle lithium ion batteries?
Yes, people can recycle lithium ion batteries. Recycling centers recover valuable metals and prevent pollution. Many stores and communities offer battery recycling programs. Always follow local rules for safe disposal.
What should people do if a battery starts to swell or leak?
People should stop using the battery right away. Move it to a safe area away from flammable items. Contact a recycling center or hazardous waste facility for safe disposal.
Why do some lithium ion batteries catch fire?
Short circuits, overheating, or damage can cause fires. Poor-quality batteries or misuse increase the risk. Safety features and proper handling help prevent most accidents.

