
The main difference between lithium and lithium-ion batteries is reusability. Lithium-ion batteries are designed for repeated charging and use. In contrast, lithium batteries are single-use power sources.
An Analogy 💡 People can think of rechargeable lithium-ion batteries like a refillable water bottle. They can be used over and over. Lithium batteries function like a disposable bottle; they are used once and then replaced.
The Core Difference Between Lithium and Lithium-ion Batteries
Understanding the key differences between these power sources starts with their chemistry. The fundamental difference between lithium and lithium-ion batteries lies in their ability to be recharged. One is designed for a single use, while the other can be used hundreds or thousands of times. This capability is determined by the chemical reactions happening inside.
What Makes a Battery Rechargeable?
A battery’s ability to recharge depends on a reversible chemical reaction. In lithium-ion batteries, the internal components are designed to allow this process to happen repeatedly. An external power source, like your phone charger, reverses the flow of energy.
This charging process involves several key steps:
- The positive material gives up electrons.
- The negative material collects these same electrons.
- An external charger drives this flow of electrons through the circuit.
- An internal substance called an electrolyte allows ions to move between the positive and negative sides, completing the circuit and restoring the battery’s charge.
This cycle can repeat many times, allowing you to use your devices over and over.
Why Primary Lithium is Single-Use
Primary lithium batteries operate on an irreversible chemical reaction. Once the battery provides its energy, the internal chemistry changes permanently. The materials cannot be returned to their original state using an external charger.
Safety Alert ⚠️ Users should never attempt to recharge single-use lithium batteries. Forcing an electrical current into them can cause overheating, leakage, or even an explosion. The battery’s chemistry is not designed to handle this reverse flow of energy.
The design of lithium batteries prioritizes a long shelf life and high energy density for a single discharge cycle. Once that cycle is complete, the battery must be disposed of properly and replaced.
Understanding Lithium-ion Batteries

Lithium-ion batteries are the powerhouses behind most modern portable electronics. Their design allows for repeated charging, providing reliable and rechargeable power.
How Li-ion Chemistry Works
The magic of a lithium-ion battery happens through the movement of lithium ions. Inside each battery are three main parts that work together.
- Anode (Negative Side): This part is typically made of carbon. It stores lithium ions when the battery is charging.
- Cathode (Positive Side): This part is a metal oxide. It releases lithium ions when the battery is charging.
- Electrolyte: This is a chemical liquid that allows ions to move between the anode and cathode.
When you use your device, lithium ions travel from the anode to the cathode, creating an electrical current. When you charge it, an external power source reverses this process, sending the ions back to the anode for storage.
Key Performance Characteristics
Lithium-ion batteries offer excellent performance for demanding devices. They have a high energy density, meaning they can store a lot of energy in a small, lightweight package. They also have a low self-discharge rate, typically losing only 3-5% of their charge per month when not in use. A key benefit is the lack of a “memory effect.” Users can charge their devices at any battery level without harming the battery’s capacity, which allows for convenient partial charging.
Types of Lithium-ion Chemistry
Not all lithium-ion batteries are the same. Different chemistries offer different trade-offs between cost, lifespan, and performance. The three most common types are Lithium Cobalt Oxide (LCO), Lithium Manganese Oxide (LMO), and Lithium Iron Phosphate (LFP).
| Feature | LCO (Lithium Cobalt Oxide) | LMO (Lithium Manganese Oxide) | LFP (Lithium Iron Phosphate) |
|---|---|---|---|
| Specific Energy | High | Moderate | Lower |
| Lifespan | Short (500-1,000 cycles) | Shorter (300-700 cycles) | Long (>2,000 cycles) |
| Safety | Concerns | Enhanced | Excellent |
| Common Use | Small electronics | Power tools, some EVs | Energy storage, EVs |
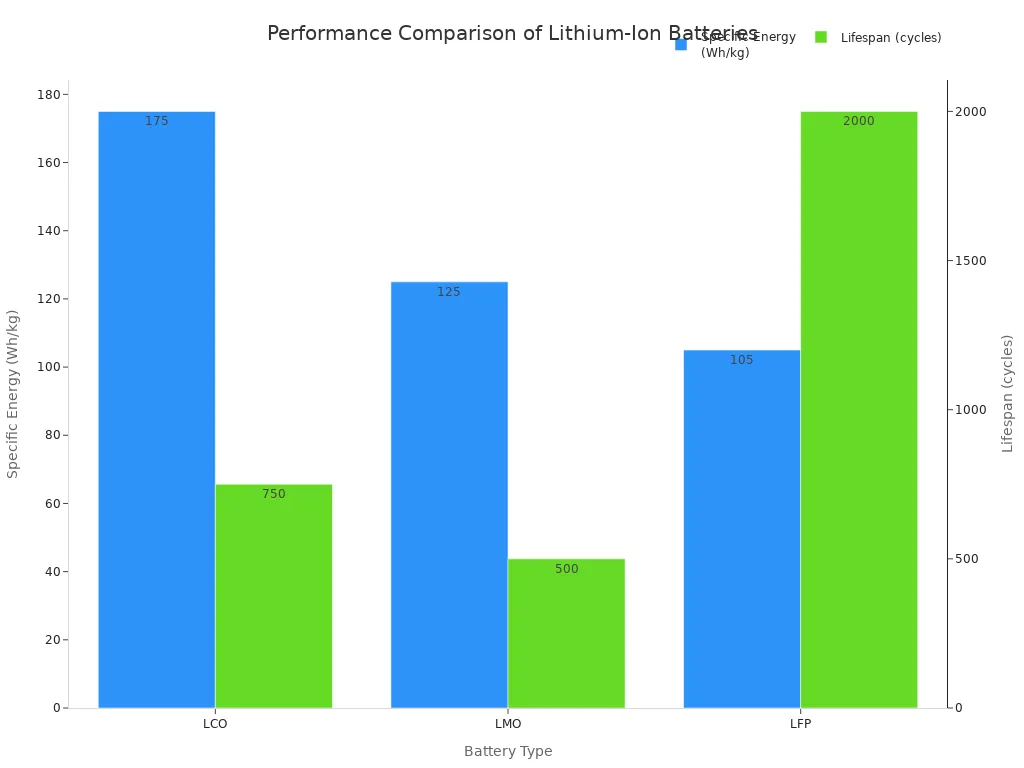
These variations allow manufacturers to choose the best type of battery for a specific product, from a smartphone to an electric bus.
Common Applications
You can find lithium-ion batteries in many devices you use every day. They power everything from smartphones, laptops, and digital cameras to larger items like cordless power tools and electric vehicles (EVs). Their ability to deliver high power makes them ideal for these applications. They are also becoming popular power storage solutions for homes with solar panels.
Lifespan and Degradation
A lithium-ion battery does not last forever. Its ability to hold a charge decreases over time with each charge-and-discharge cycle. Typically, a battery shows significant capacity loss after about 500 cycles. This gradual degradation is why your phone battery does not seem to last as long after a couple of years.
Safety and Maintenance
Proper care ensures stable performance and safety. Modern batteries include safety mechanisms to prevent overcharging, but user habits are also important.
Safety Tips 💡
- Always use the official charger for your device.
- Avoid charging batteries in extreme heat or cold.
- Store batteries at room temperature and away from flammable materials.
- If you see a battery swelling or leaking, stop using it immediately and dispose of it properly at a designated recycling center.
A Closer Look at Primary Lithium Batteries
Primary lithium batteries are single-use power sources known for their long life and reliability. Unlike their rechargeable counterparts, their internal chemistry is designed for one complete discharge cycle.
How Lithium Metal Chemistry Works
The power generation inside primary lithium batteries comes from an irreversible chemical reaction. The battery contains a few key parts:
- Anode: The negative side, made of pure lithium metal.
- Cathode: The positive side, often made of manganese dioxide.
- Electrolyte: A salt solution that allows ions to move internally.
When you use the battery, lithium atoms at the anode give up electrons. These electrons travel through your device to the cathode, creating the electrical current that provides power. This process permanently changes the battery’s internal materials, which is why it cannot be recharged.
Key Performance Characteristics
Primary lithium batteries offer excellent performance for specific needs. Their main advantage is a very high energy density, allowing them to pack a lot of power into a small size. They also function well in a wide range of temperatures, from extreme cold to high heat. This reliable performance makes them a top choice for critical applications where changing batteries is difficult or infrequent.
Common Applications
You will find these powerful cells in many low-drain devices that require long-term, dependable energy. Common examples include:
- Watches and key fobs
- Remote controls
- Smoke alarms and carbon monoxide detectors
- Medical devices like pacemakers
Exceptional Shelf Life
One of the most impressive features of lithium batteries is their exceptional shelf life. They can sit in storage for 10 to 20 years and still retain most of their original charge. This makes them perfect for emergency equipment, such as flashlights or backup power systems, that must work after long periods of inactivity.
Safety and Disposal
Proper handling and disposal are crucial for safety.
Important Safety Note ⚠️ Never throw lithium batteries in your household trash. They are considered hazardous waste and can cause fires if damaged. Always take them to a designated battery recycling center or a household hazardous waste facility.
When preparing for disposal, place tape over the battery terminals. This simple step prevents them from accidentally short-circuiting. Proper storage in a cool, dry place also helps prevent potential hazards.
Head-to-Head Comparison

Choosing the right battery depends on understanding its specific strengths. This section compares lithium-ion and primary lithium batteries across key performance metrics.
Energy Density and Voltage
Both battery types offer high energy density, meaning they pack a lot of power into a small space. However, their voltage levels differ. A standard lithium-ion cell provides a nominal voltage of around 3.6V to 3.7V. In contrast, most primary lithium batteries, like coin cells, operate at 3V.
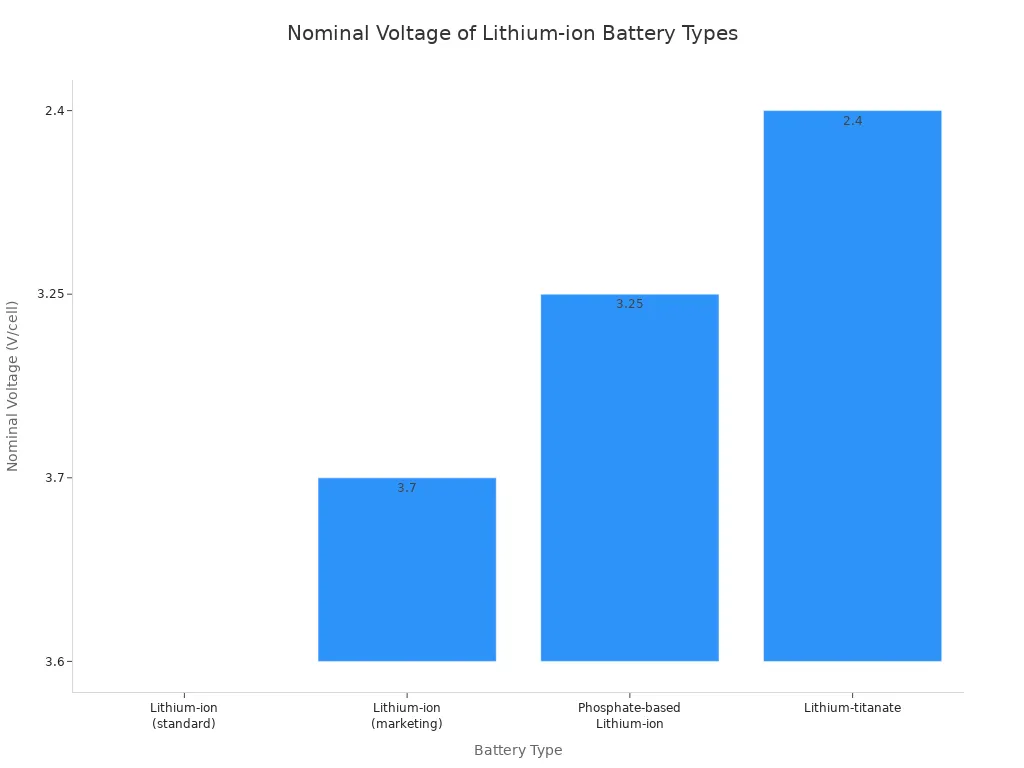
The voltage of lithium-ion batteries can also vary based on their specific chemistry.
| Battery Type | Nominal Voltage |
|---|---|
| Lithium-ion (Standard) | 3.6V |
| Lithium Iron Phosphate (LFP) | 3.2V |
| Primary Lithium (Coin Cell) | 3.0V |
Cost: Upfront vs. Long-Term
Primary lithium batteries have a high upfront cost for a single-use product. Lithium-ion batteries cost more to purchase initially, but their reusability makes them more economical over time.
Long-Term Value 💰 The ability to recharge a lithium-ion battery hundreds of times significantly lowers its cost per use. This makes it a cost-effective choice for frequently used devices.
Temperature Performance
Temperature affects battery performance. Lithium-ion batteries work best in moderate conditions. Extreme heat or cold can reduce their efficiency and lifespan. Primary lithium batteries generally handle a wider temperature range, especially extreme cold, making them more reliable for outdoor or emergency devices.
| Li-ion Operation | Optimal Temperature Range |
|---|---|
| Charging | 0°C to 45°C (32°F to 113°F) |
| Discharging (Use) | -20°C to 60°C (-4°F to 140°F) |
Self-Discharge Rate
Self-discharge is the rate at which a battery loses charge while not in use. Lithium-ion batteries typically lose about 1-2% of their charge per month after an initial drop. Primary lithium batteries excel in this area, losing only about 10% of their charge over five years. This makes them perfect for long-term storage in devices like smoke detectors and emergency flashlights.
Which Battery Should You Choose?
Selecting the correct battery technology depends entirely on the device’s power needs. A high-power drill requires a different energy source than a simple TV remote. Understanding these differences helps consumers make the best choice for performance and value.
For High-Drain Electronics
High-drain electronics demand a lot of power quickly and consistently. Devices like smartphones, laptops, cordless power tools, and electric vehicles fall into this category. For these applications, lithium-ion batteries are the superior choice. Their chemistry is designed for high performance and reusability.
Several key features make them ideal for power-hungry devices:
- High Energy Density: They store more energy in a smaller, lighter package. This is crucial for portable electronics and for maximizing the range of electric vehicles.
- Longevity: High-quality lithium-ion batteries can endure hundreds of charge cycles. This long-term use reduces replacement frequency, making them cost-effective and more sustainable.
- Efficiency: These batteries have excellent charge and discharge efficiency. They waste very little energy, delivering powerful and consistent output for demanding tasks.
- High-Current Discharge: They can provide a strong burst of energy when needed. This explosive power is perfect for power tools and for the acceleration of vehicles.
Power tools, for example, need a battery that can deliver a significant amount of work on a single charge. The high energy density of lithium-ion batteries allows them to power these demanding tools far longer than older battery types.
For Low-Drain Devices
Low-drain devices consume very little power over a long period. Think of watches, remote controls, key fobs, and smoke detectors. These items need a reliable power source that can last for years without replacement. Primary lithium batteries are the perfect fit for this job.
Why Choose Lithium for Low-Drain? 🤔 Their main advantage is reliability over time. You can install them and forget about them for years, knowing they will work when needed.
Primary lithium batteries offer several distinct advantages for these applications:
- Exceptional Shelf Life: They can be stored for 10 to 20 years and still hold most of their charge.
- Minimal Self-Discharge: These batteries lose less than 1% of their charge per year. This makes them ideal for emergency devices like smoke alarms.
- Wide Temperature Range: They operate effectively in extreme cold and heat, ensuring reliability in various environments.
- High Reliability: Their consistent, low-power output is essential for safety equipment and medical devices where failure is not an option.
The chemistry of lithium batteries, such as lithium-manganese dioxide (LiMnO2), is affordable and provides a very long service life, making it a dominant choice for these single-use applications.
Making an Informed Choice
Making the right choice comes down to evaluating a few key factors. Before you buy, consider the device’s function, your usage patterns, and the long-term cost.
A simple checklist can guide your decision:
- Device Power Needs: Does the device need a lot of power quickly (high-drain), or does it need a little power over many years (low-drain)?
- Usage Frequency: Will you use the device daily and need to recharge it, or will it sit for long periods until needed?
- Cost: Are you willing to pay more upfront for a rechargeable battery that saves money over time, or do you need a cheaper, single-use option?
- Safety and Environment: Consider the proper disposal methods for single-use batteries versus the extended life of rechargeable ones.
The following table summarizes the key trade-offs to help you decide.
| Factor | Choose Lithium-ion If… | Choose Primary Lithium If… |
|---|---|---|
| Primary Use | You have high-drain devices like phones, laptops, or power tools. | You have low-drain devices like remotes, watches, or smoke alarms. |
| Reusability | You need a rechargeable battery for frequent use. | You need a single-use battery for long-term, reliable power. |
| Cost Model | You prefer a higher upfront cost for better long-term value. | You prefer a lower upfront cost for an infrequent purchase. |
| Shelf Life | Short-term storage is sufficient. | You need the battery to work after years in storage. |
Ultimately, matching the battery to the application ensures optimal performance, safety, and value.
Choosing the right battery is simple. People should select lithium-ion for devices they charge daily and primary lithium for items needing power over many years. Lithium-ion offers reusability, while primary lithium batteries provide an exceptional shelf life. As technology advances, new options like solid-state and sodium-ion batteries promise even better performance. These future batteries aim for higher energy density and improved safety, continuing the evolution of portable power.
The Future of Batteries 🔮 Solid-state batteries may soon offer major improvements over today’s technology.
| Feature | Solid-State Batteries (SSB) | Lithium-Ion Batteries (LIB) |
|---|---|---|
| Energy Density | 350–700 Wh/kg | 150–300 Wh/kg |
| Safety | Much safer | Risk of fire |
| Charging Speed | 12–15 min to 80% | 30–45 min to 80% |
Making informed choices helps ensure both device performance and sustainability.
FAQ
### What is the main difference between lithium and lithium-ion?
The key difference is reusability. Lithium-ion batteries are rechargeable, making them suitable for frequent use in devices like phones. Primary lithium batteries are single-use and not rechargeable. People replace them after one full discharge cycle.
### Can people recycle both types of batteries?
Yes, recycling is important for both. ♻️ Lithium-ion batteries contain valuable materials that can be recovered. Primary lithium batteries are hazardous waste. Users should take both types to a designated e-waste or battery recycling center for safe and proper disposal.
### Are there rules for flying with these batteries?
Airlines have strict rules for lithium batteries. ✈️ People must carry devices with lithium-ion batteries and spare batteries in their carry-on luggage. The FAA sets limits on battery size and quantity. Always check with your airline before traveling.
### Why does a phone battery lose power over time?
Lithium-ion batteries degrade with each charge cycle. This process reduces their total capacity. After about two years of regular use, a phone battery will not hold its charge as long as it did when new. This gradual capacity loss is normal.

